초록
Background
Polymerase chain reaction (PCR) methods from direct specimen are widely used for the rapid and accurate detection of mycobacteria infection. In this study, we evaluated two domestically developed detection kits for Mycobacterium tuberculosis (MTB) and nontuberculous mycobacteria (NTM) using re-al-time PCR.
Methods
A total of 348 samples from patients with suspected tuberculosis were tested with real-time PCR over seven months. We performed real-time PCR using the recently developed Anyplex plus MTB/NTM Detection kit (Seegene) with the CFX 96TM Real- time PCR System (Bio-Rad Laboratories) and the conventional AdvanSure TB/NTM real-time PCR kit (LG Life Sciences) with the SLAN Real-time PCR detection system (LG Life Sciences) to evaluate their performance for detecting MTB and NTM.
Results
The two real-time PCR systems showed 96.8% concordance rate for MTB-positive, NTM-positive, and negative results. Based on culture results, the sensitivity and specificity for the detection of MTB using PCR were 71.0% and 94.9% for Anyplex plus, and 78.1% and 93.9% for AdvanSure, respectively. For the detection of NTM, the sensitivity and specificity were 33.3% and 98.4% for Anyplex plus, and 51.7% and 97.9% for AdvanSure. Both PCR systems showed high MTB positive results in bronchial wash-ing and sputum samples.
Go to : 
REFERENCES
1.Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 2nd ed.Korea Centers for Disease Control and Prevention;2014. p. 30–1.
2.Jang MA., Shin SY., Park S., Seong MW., Park SS., Kim SH. Diagnostic Genetics Subcommittee, The Korean Association of Quality Assurance for Clinical Laboratory. Annual report on the external quality assessment of diagnostic genetics in Korea (2013). J Lab Med Qual Assur. 2014. 36:71–83.

3.Korea Centers for Disease Control and Prevention. Manual of Laboratory Tests for Tuberculosis. Korea Centers for Disease Control and Prevention. 2013. 75–80.
4.Joint Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 2nd ed.Korea Centers for Disease Control and Prevention;2014. p. 227–8.
5.Shinnick TM., Iademarco MF., Ridderhof JC. National plan for reliable tuberculosis laboratory services using a systems approach. Recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services.
6.Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009. 58:7–10.
7.Broccolo F., Scarpellini P., Locatelli G., Zingale A., Brambilla AM., Cichero P, et al. Rapid diagnosis of mycobacterial infections and quantitation of Mycobacterium tuberculosis load by two real-time calibrated PCR assays. J Clin Microbiol. 2003. 41:4565–72.
8.Chang HE., Heo SR., Yoo KC., Song SH., Kim SH., Kim HB, et al. Detection of Mycobacterium tuberculosis complex using real-time polymerase chain reaction. Korean J Lab Med. 2008. 28:103–8.
9.Lim J., Kim J., Kim JW., Ihm C., Sohn YH., Cho HJ, et al. Multicenter evaluation of Seegene Anyplex TB PCR for the detection of Mycobacterium tuberculosis in respiratory specimens. J Microbiol Biotechnol. 2014. 24:1004–7.
10.Lee JH., Kim BH., Lee MK. Performance evaluation of anyplex plus MTB/NTM and MDR-TB detection kit for detection of mycobacteria and for antituberculosis drug susceptibility test. Ann Clin Microbiol. 2014. 17:115–22.

11.Lee H., Park KG., Lee G., Park J., Park YG., Park YJ. Assessment of the quantitative ability of AdvanSure TB/NTM real-time PCR in respiratory specimens by comparison with phenotypic methods. Ann Lab Med. 2014. 34:51–5.

12.Cho SY., Kim MJ., Suh JT., Lee HJ. Comparison of diagnostic performance of three real-time PCR kits for detecting Mycobacterium species. Yonsei Med J. 2011. 52:301–6.
Go to : 
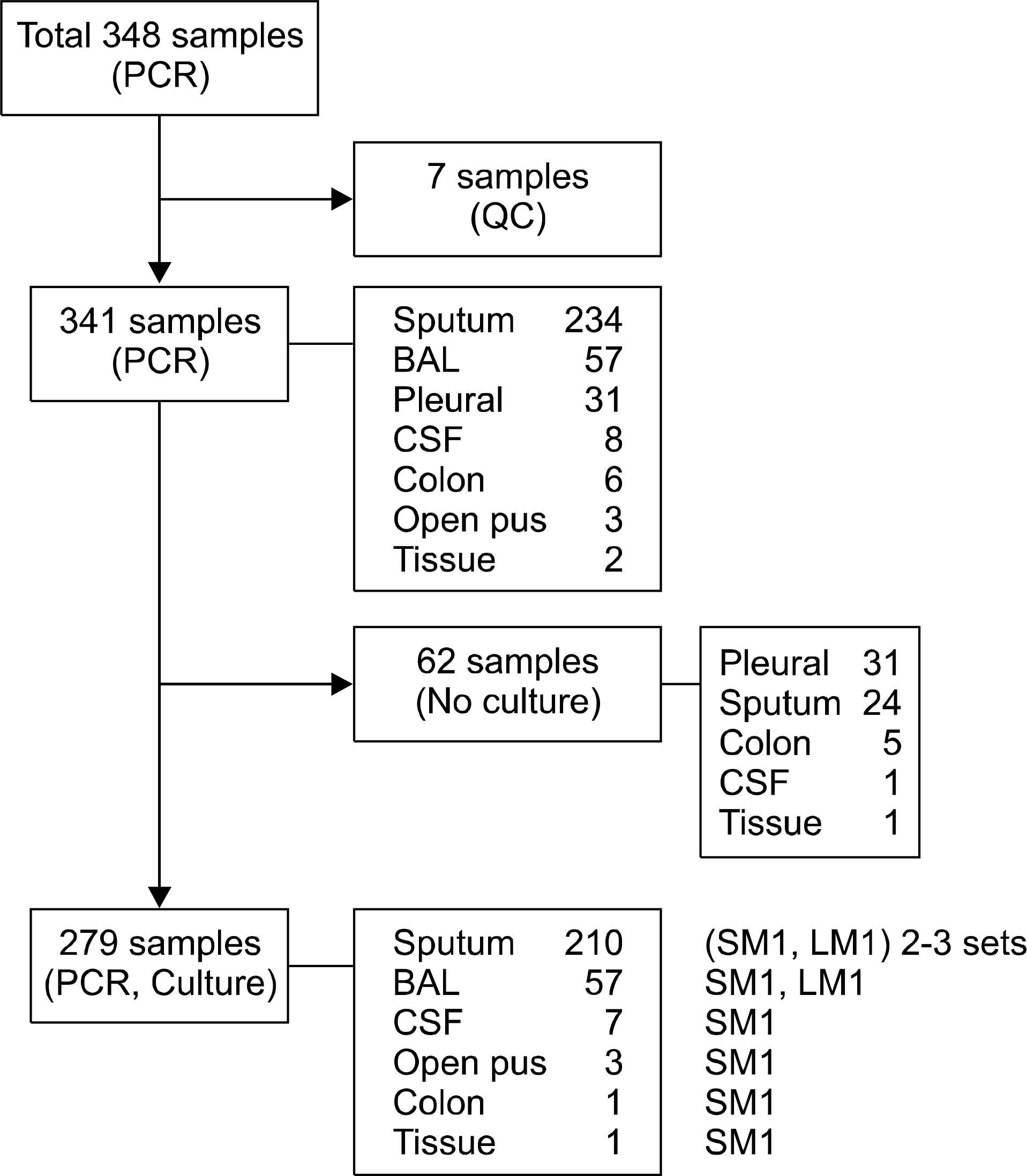 | Fig. 1.Type distributions of samples according to the tests performed. Abbreviations: BAL, bronchoalveolar lavage; Pleural, pleural fluid; CSF, cerebrospinal fluid, SM, solid media; LM, liquid media. |
Table 1.
Results of two real-time PCR systems according to AFB smear and culture results
| Target | AFB smear | Assays | Culture + (79) | Culture − (200) | Sensitivity/Specificity | PPV/NPV | ||
|---|---|---|---|---|---|---|---|---|
| PCR+ | PCR− | PCR+ | PCR− | |||||
| MTB | Positive | Anyplex plus | 23 | 1 | 0 | 2 | 95.8/100.0 | 100.0/66.7 |
| AdvanSure | 23 | 0 | 0 | 2 | 100.0/100.0 | 100.0/100.0 | ||
| Negative | Anyplex plus | 25 | 15 | 10 | 185 | 62.5/94.9 | 71.4/92.5 | |
| AdvanSure | 25 | 11 | 12 | 182 | 69.4/93.8 | 67.6/94.3 | ||
| All∗ | Anyplex plus | 49† | 20§ | 10 | 187 | 71.0/94.9 | 83.1/90.3 | |
| AdvanSure | 50‡ | 14∥ | 12 | 184 | 78.1/93.9 | 80.6/92.9 | ||
| NTM | Positive | Anyplex plus | 9 | 1 | 0 | 2 | 90.0/100.0 | 100.0/66.7 |
| AdvanSure | 10 | 0 | 0 | 2 | 100.0/100.0 | 100.0/100.0 | ||
| Negative | Anyplex plus | 1 | 15 | 3 | 185 | 6.3/98.4 | 25.0/92.5 | |
| AdvanSure | 5 | 11 | 4 | 182 | 31.3/97.8 | 55.6/94.3 | ||
| All∗ | Anyplex plus AdvanSure | 1015 | 20§14∥ | 3 4 | 187184 | 33.3/98.4 51.7/97.9 | 76.9/90.3 78.9/92.9 | |
Table 2.
MTB positive rates according to sample types in culture and two real-time PCR systems
| Specimen | No. MTB positive (%) | ||
|---|---|---|---|
| Culture | Anyplex plus | AdvanSure | |
| Pulmonary specimen | 53/267 (19.9%) | 58/267 (21.7%) | 60/267 (22.5%) |
| Extrapulmonary specimen∗ | 4/12 (33.3%) | 1/12 (8.3%) | 2/12 (16.7%) |
| Total | 57/279 (20.4%) | 59/279 (22.1%) | 65/279 (22.2%) |
Table 3.
Results of two real-time PCR systems according to culture results
| Anyplex plus PCR | AdvanSure PCR | Number | |
|---|---|---|---|
| Culture + (79) | + | + | 59 |
| + | − | 0 | |
| − | + | 6 | |
| − | − | 14 | |
| Culture − (200) | + | + | 13 |
| + | − | 0 | |
| − | + | 3 | |
| − | − | 184 |
Table 4.
Result correlations of two real-time PCR systems
| Anyplex plus | |||||
|---|---|---|---|---|---|
| MTB | NTM | Negative | Total | ||
| AdvanSure | MTB | 61 | 0 | 4 | 65 |
| NTM | 1 | 13 | 5 | 19 | |
| Negative | 1 | 0 | 256 | 257 | |
| Total | 63 | 13 | 265 | 341 | |
Table 5.
Result summary for samples with discrepant results among test methods including culture, AFB smear, and PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download