초록
Background
For early diagnosis and treatment of influenza, rapid influenza diagnostic tests are commonly used. We evaluated the performance of the BD Veritor System for Rapid Detection of Flu A+ B (BD Veritor System; BD Diagnostics, USA) compared to multiplex real-time RT-PCR.
Methods
A total of 3,213 nasal and nasopharyngeal swab specimens in transport media from patients with influenza-like symptoms were tested with the BD Veritor System from December 2013 to April 2014. The sensitivity and specificity of 127 specimens were determined simultaneously using multiplex real-time RT- PCR with the AdvanSure RV real-time PCR (AdvanSure PCR; LG Life Sciences, Korea).
Results
Influenza viruses were detected in 41.3% (1,327/3,213) of all specimens tested using the BD Veritor System. Of the 127 specimens, 27 influenza A and 17 influenza B viruses were identified by the AvanSure PCR. The sensitivity and specificity of the BD Veritor System relative to the AdvanSure PCR was 85.2% and 99.0% for influenza A, and 58.8% and 99.1% for influenza B. Of the 190 specimens that tested negative using the BD Veritor System, the AdvanSure PCR detected influenza A and influenza B in 19 and 13 specimens, respectively. The mean threshold cycle (Ct) values of the antigen positive specimens were lower than those of the antigen negative specimens.
Conclusion
The BD Veritor System showed excellent specificity for both influenza types and good sensitivity for influenza A. However, the system was less sensitive for influenza B compared to multiplex re-al-time RT-PCR. For accurate diagnosis of false negative specimens, a molecular diagnostic test should be performed. The BD Veritor system could be a use-ful tool for screening and early diagnosis of influenza.
Go to : 
REFERENCES
1.Fiore AE., Uyeki TM., Broder K., Finelli L., Euler GL., Singleton JA, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010. 59:1–62.
2.Korean Centers for Disease Control & Prevention. Weekly Sentinel Surveillance Report. http://www.cdc.go.kr/CDC/info/CdcKrInfo0402.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU 0045/. [Online] (last visited on 31 December 2014).
3.Thompson WW., Shay DK., Weintraub E., Brammer L., Bridges CB., Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004. 292:1333–40.

4.Wie SH., So BH., Song JY., Cheong HJ., Seo YB., Choi SH, et al. A comparison of the clinical and epidemiological characteristics of adult patients with laboratory-confirmed influenza A or B during the 2011-2012 influenza season in Korea: a multi-center study. PLoS One. 2013. 8:e62685.

5.Kumar S., Henrickson KJ. Update on influenza diagnostics: lessons from the novel H1N1 influenza A pandemic. Clin Microbiol Rev. 2012. 25:344–61.

6.BD Diagnostics. BD Veritor System for Rapid Detection of Flu A+B. Spark, MD; BD Diagnostics. 2014.
7.Hwang Y., Kim K., Lee M. Evaluation of the efficacies of rapid antigen test, multiplex PCR, and real-time PCR for the detection of a novel influenza A (H1N1) virus. Korean J Lab Med. 2010. 30:147–52.

8.Chartrand C., Leeflang MM., Minion J., Brewer T., Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012. 156:500–11.
9.Hassan F., Nguyen A., Formanek A., Bell JJ., Selvarangan R. Comparison of the BD Veritor System for Flu A+B with the Alere BinaxNOW influenza A&B card for detection of influenza A and B viruses in respiratory specimens from pediatric patients. J Clin Microbiol. 2014. 52:906–10.
10.Nam MH., Jang JW., Lee JH., Cho CH., Lim CS., Kim WJ. Clinical performance evaluation of the BD Veritor System Flu A+B assay. J Virol Methods. 2014. 204:86–90.

11.Kwon D., Shin K., Kwon M., Oh HB., Kang C., Lee JY. Deve-lopment and evaluation of a rapid influenza diagnostic test for the pandemic (H1N1) 2009 influenza virus. J Clin Microbiol. 2011. 49:437–8.

12.Korean Centers for Disease Control & Prevention. Public Health Weekly Report. Rapid diagnostic tests for influenza.http://www.cdc.go.kr/CDC/contents/CdcKrContentLink.jsp?fid.=31&cid=12744&ctype=6/. [Online] (last visited on 23 January 2015).
13.LG Life Sciences. AdvanSure RV real-time PCR. Seoul, Korea: LG Life Sciences;2013.
14.Fiore AE., Fry A., Shay D., Gubareva L., Bresee JS., Uyeki TM. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011. 60:1–24.
15.Carrat F., Vergu E., Ferguson NM., Lemaitre M., Cauchemez S., Leach S, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008. 167:775–85.

16.Ginocchio CC., Zhang F., Manji R., Arora S., Bornfreund M., Falk L, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009. 45:191–5.

17.Monto AS., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000. 160:3243–7.

18.Hsu J., Santesso N., Mustafa R., Brozek J., Chen YL., Hopkins JP, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012. 156:512–24.
19.Muthuri SG., Myles PR., Venkatesan S., Leonardi-Bee J., Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A (H1N1) pandemic: a systematic review and meta- analysis in hospitalized patients. J Infect Dis. 2013. 207:553–63.
Go to : 
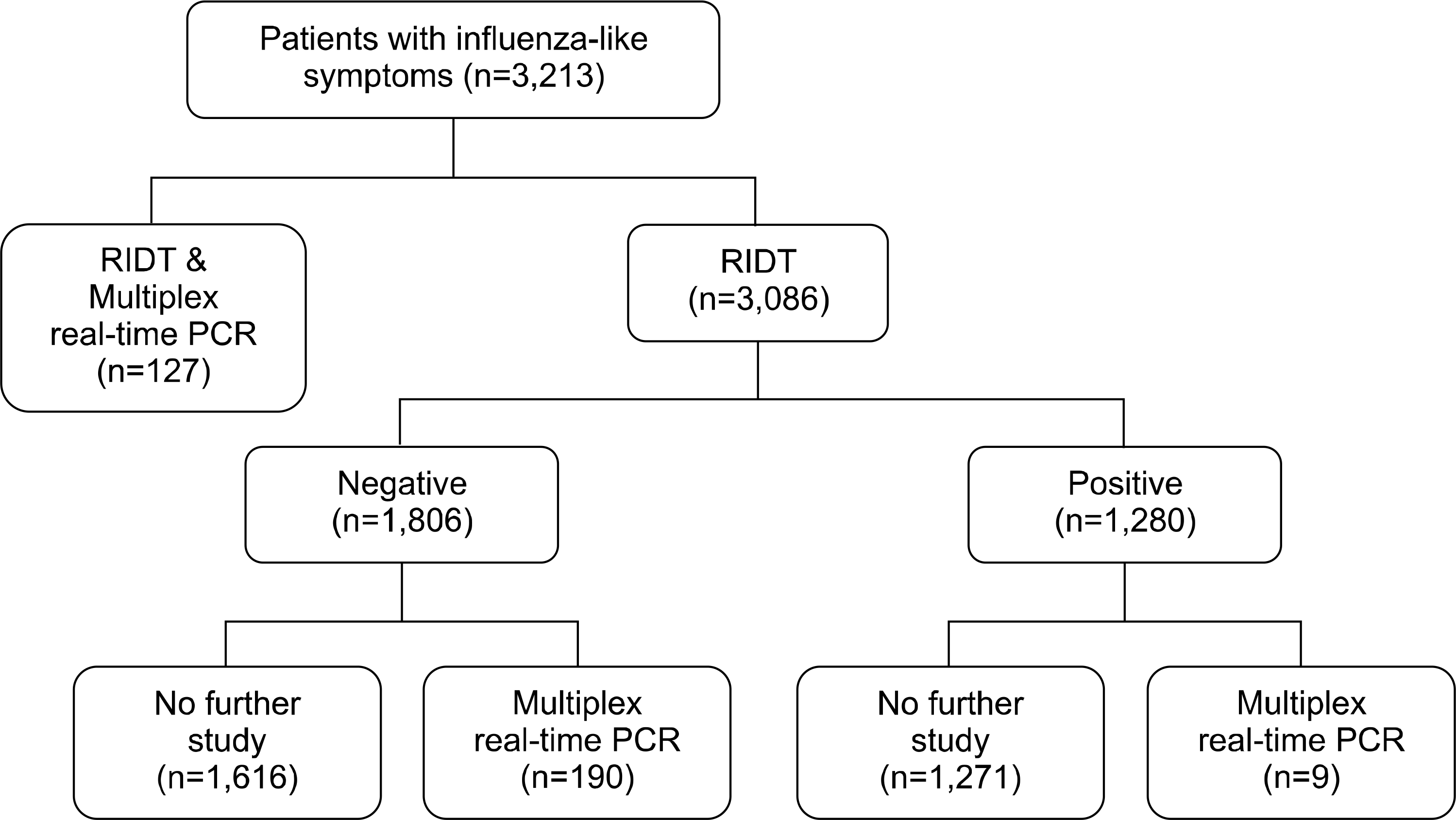 | Fig. 1.Flow diagram of the cate-gorization of the patients. Abbreviation: RIDT, rapid influenza diagnostic test. |
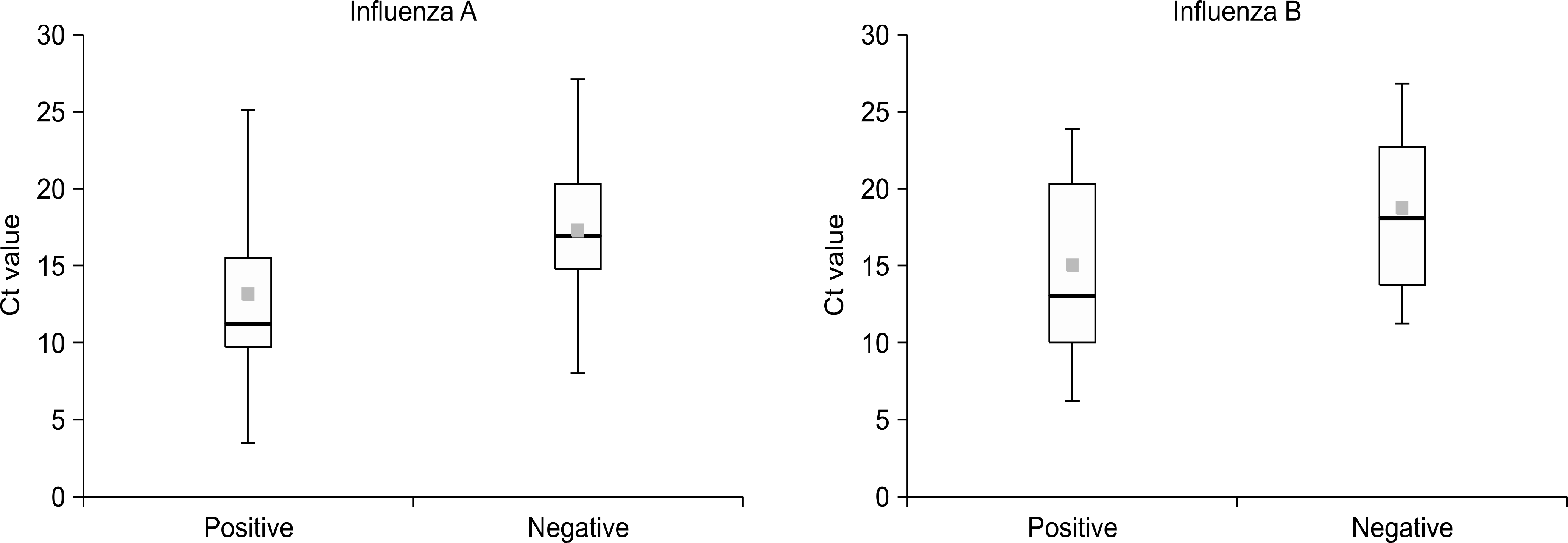 | Fig. 2.Comparison of the Ct values of the antigen positive specimens and the antigen negative specimens for influenza A and influenza B. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download