초록
Background
Nasopharyngeal aspirate (NPA) is known as the best specimen for accurate diagnosis of viral respiratory infections in pediatric patients, but the procedure is very annoying. Recently introduced flocked swabs have been reported to be easy to obtain a good quality specimen and comfortable to patients. The purpose of this study was to compare the sensitivities between NPA and nasopharyngeal flocked swabs (NPFS) for detection of respiratory viruses in children.
Methods
For this study, 111 hospitalized children with acute respiratory tract infections were recruited. NPA and NPFS were performed in parallel from each patient. NPFS were always collected after NPA. Specimens were tested for six common respiratory viruses in triplicate using indirect immunofluorescence (IIF), viral cultures, and multiplex reverse transcription PCR (RT- PCR).
Results
The proportion of specimens inadequate for IIF was higher in NPA (23.4%) than NPFS (5.4%). According to the consensus positive, the positive rates of NPFS were higher than those of NPA when using IIF (45.7% and 30.6%, P=0.048) and culture (38.7% and 27.9%, P=0.004). However, the false-positive rates of NPFS were higher than those of NPA when using IIF (12.4% and 1.2%, P=0.004). The positive rates of NPFS and those of NPA were not different in multiplex RT-PCR (67.6% and 55.9%, P=0.055).
REFERENCES
2.Bourgeois FT., Valim C., Wei JC., McAdam AJ., Mandl KD. In-fluenza and other respiratory virus-related emergency department visits among young children. Pediatrics. 2006. 118:e1–8.

3.Park JS. Acute viral lower respiratory tract infections in children. Korean J Pediatr. 2009. 52:269–76.

4.Li H., McCormac MA., Estes RW., Sefers SE., Dare RK., Chappell JD, et al. Simultaneous detection and high-throughput identifi-cation of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007. 45:2105–9.

5.Vega R. Rapid viral testing in the evaluation of the febrile infant and child. Curr Opin Pediatr. 2005. 17:363–7.

6.Meerhoff TJ., Houben ML., Coenjaerts FE., Kimpen JL., Hofland RW., Schellevis F, et al. Detection of multiple respiratory pathogens during primary respiratory infection: nasal swab versus nasopharyngeal aspirate using real-time polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2010. 29:365–71.

7.Moore C., Corden S., Sinha J., Jones R. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real-time NASBA. J Virol Methods. 2008. 153:84–9.

8.Debyle C., Bulkow L., Miernyk K., Chikoyak L., Hummel KB., Hen-nessy T, et al. Comparison of nasopharyngeal flocked swabs and nasopharyngeal wash collection methods for respiratory virus detection in hospitalized children using real-time polymerase chain reaction. J Virol Methods. 2012. 185:89–93.

9.Abu-Diab A., Azzeh M., Ghneim R., Ghneim R., Zoughbi M., Tur-kuman S, et al. Comparison between pernasal flocked swabs and nasopharyngeal aspirates for detection of common respiratory viruses in samples from children. J Clin Microbiol. 2008. 46:2414–7.

10.Esposito S., Molteni CG., Daleno C., Valzano A., Cesati L., Gualtieri L, et al. Comparison of nasopharyngeal nylon flocked swabs with universal transport medium and rayon-bud swabs with a sponge reservoir of viral transport medium in the diagnosis of paediatric influenza. J Med Microbiol. 2010. 59:96–9.

11.Spyridaki IS., Christodoulou I., de Beer L., Hovland V., Kurowski M., Olszewska-Ziaber A, et al. Comparison of four nasal sampling methods for the detection of viral pathogens by RT-PCR-A GA(2)LEN project. J Virol Methods. 2009. 156:102–6.

12.Macfarlane P., Denham J., Assous J., Hughes C. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005. 90:634–5.

13.Chan KH., Peiris JS., Lim W., Nicholls JM., Chiu SS. Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J Clin Virol. 2008. 42:65–9.

15.Landry ML., Ferguson D. SimulFluor respiratory screen for rapid detection of multiple respiratory viruses in clinical specimens by immunofluorescence staining. J Clin Microbiol. 2000. 38:708–11.

16.Sung H., Park SJ., Woo YD., Choi BH., Kim MN. Evaluation of Seeplex RV detection kit for detecting rhinovirus, human metapneumovirus, and coronavirus. Korean J Lab Med. 2008. 28:109–17.
17.Ohrmalm L., Wong M., Rotzén-Östlund M., Norbeck O., Broliden K., Tolfvenstam T. Flocked nasal swab versus nasopharyngeal aspirate for detection of respiratory tract viruses in immunocompromised adults: a matched comparative study. BMC Infect Dis. 2010. 10:340.

18.Walsh P., Nguyen TA., Higashida K., Michaelson S., Pham K., Nguyen P, et al. Do infants and toddlers prefer nasal swabs or washes for specimen collection? Pediatr Infect Dis J. 2010. 29:1156–7.

19.Faden H. Comparison of midturbinate flocked-swab specimens with nasopharyngeal aspirates for detection of respiratory viruses in children by the direct fluorescent antibody technique. J Clin Microbiol. 2010. 48:3742–3.

20.Fernie BF., Gerin JL. The stabilization and purification of respiratory syncytial virus using MgSO4. Virology. 1980. 106:141–4.
21.Collins PL., Karron RA. Respiratory syncytial virus and metapneumovirus. Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2013. 1087.
Fig. 1.
Distribution of 80 respiratory viruses from 79 patients. Respiratory syncytial viruses (RSV), influenza viruses (Flu), adenovi-ruses (ADV), and parainfluenza viruses (PIV) were counted for the prevalence in this study.
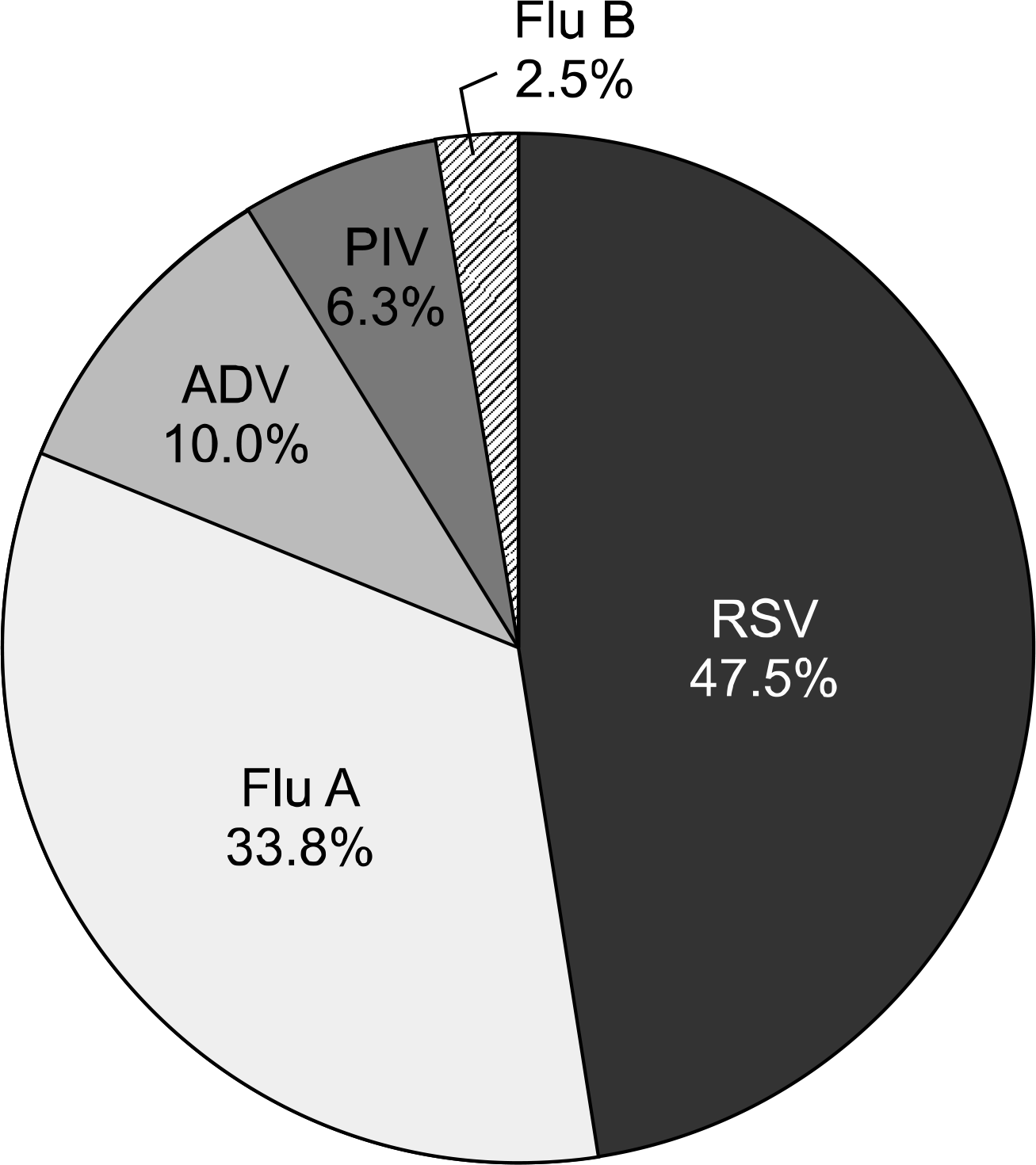
Table 1.
Consensus positive results of the triplicate examination of the nasopharyngeal aspirates (NPA) and nasopharyngeal flocked swab (NPFS) specimens for the detection of respiratory syncytial virus (RSV), influenza A virus (Flu A), influenza B virus (Flu B), parainfluenza virus (PIV)-1,-2, -3, and adenovirus (ADV)
| Interpretation | IIF* | Culture† | Multiplex RT-PCR‡ | |||
|---|---|---|---|---|---|---|
| NPA | NPFS | NPA | NPFS | NPA | NPFS | |
| Positive | 30.6% (26/85) | 45.7% (48/105) | 27.9% (31/111) | 38.7% (43/111) | 55.9% (62/111) | 67.6% (75/111) |
| Negative | 69.4% (59/85) | 54.3% (57/105) | 72.2% (80/111) | 61.3% (68/111) | 44.1% (49/111) | 32.4% (36/111) |
Table 2.
Summary of 28 discrepant results between specimens and/or detection methods according to the ‘consensus positive’




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download