초록
Background
The purpose of this study was to evaluate the effectiveness of Xpert MTB/RIF (Cepheid, USA) in the detection of Mycobacterium tuberculosis and to determine rifampin resistance.
Methods
The literature review covered the period from 16 August 2011 to 1 October 2011, and eight domestic databases and foreign databases including Ovid-Medline, Embase, and Cochrane Library were used. Key words, such as ‘Rifampin, Polymerase Chain Reaction,’ ‘GeneXpert’ and ‘Xpert MTB-RIF’ were used to search a total of 1,385 documents. The SIGN (Scottish Intercollegiate Guidelines Network) tool was used to evaluate the quality of the 20 selected studies.
Results
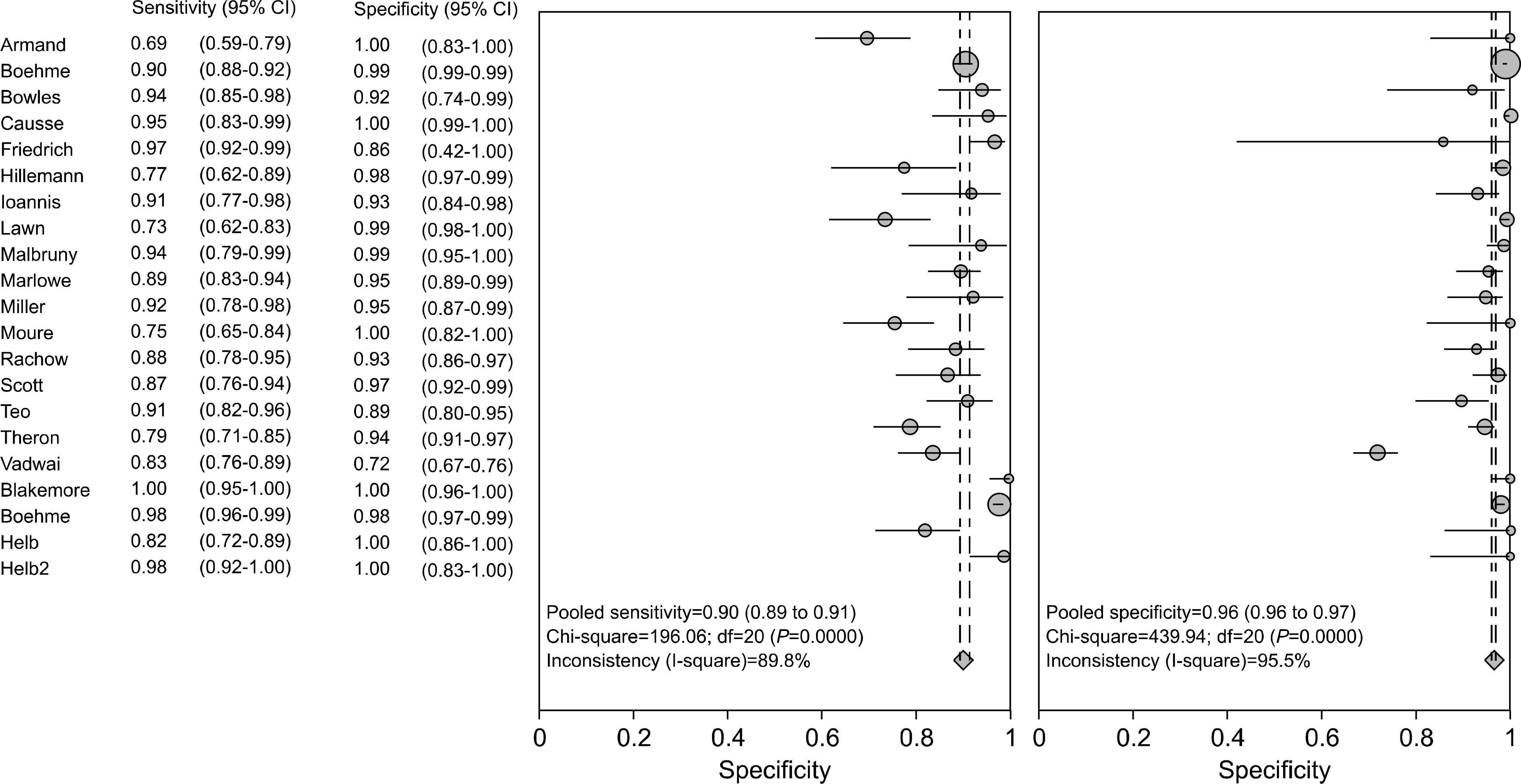
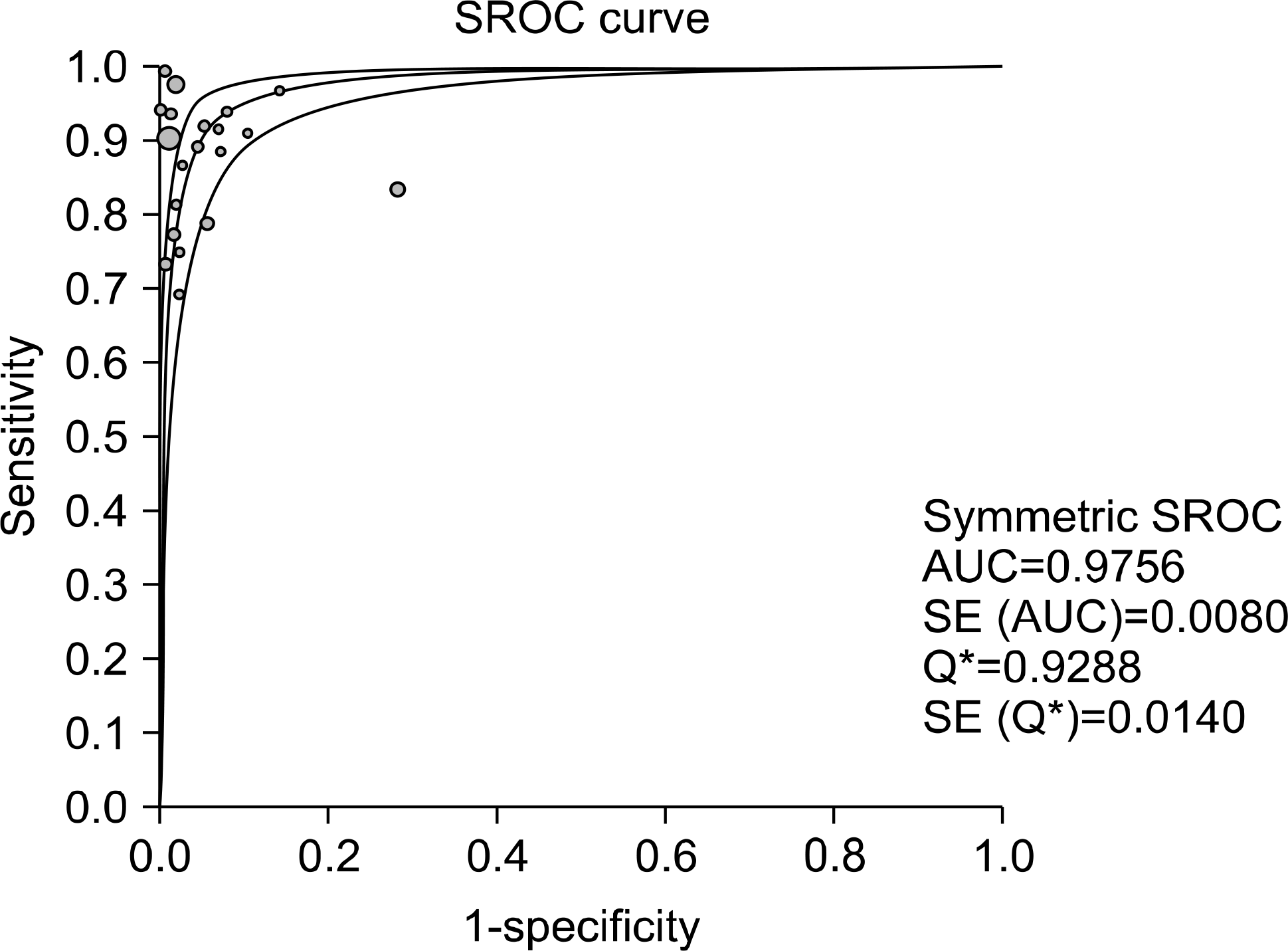
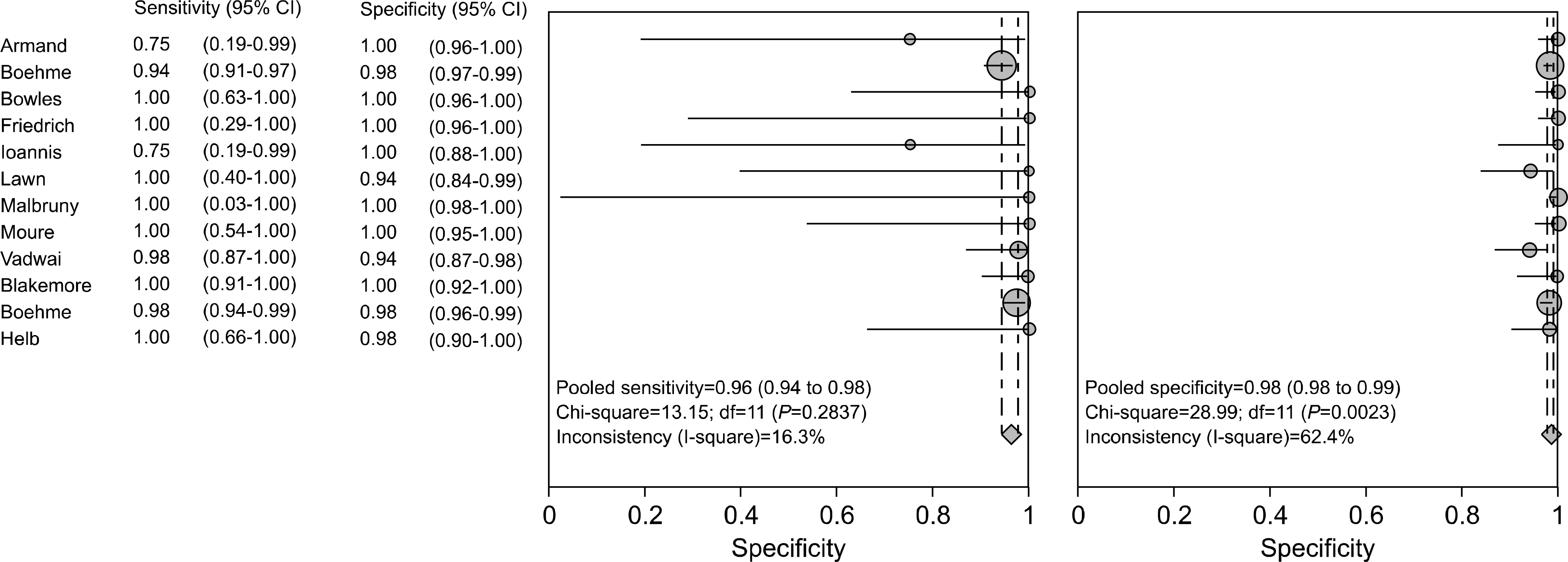
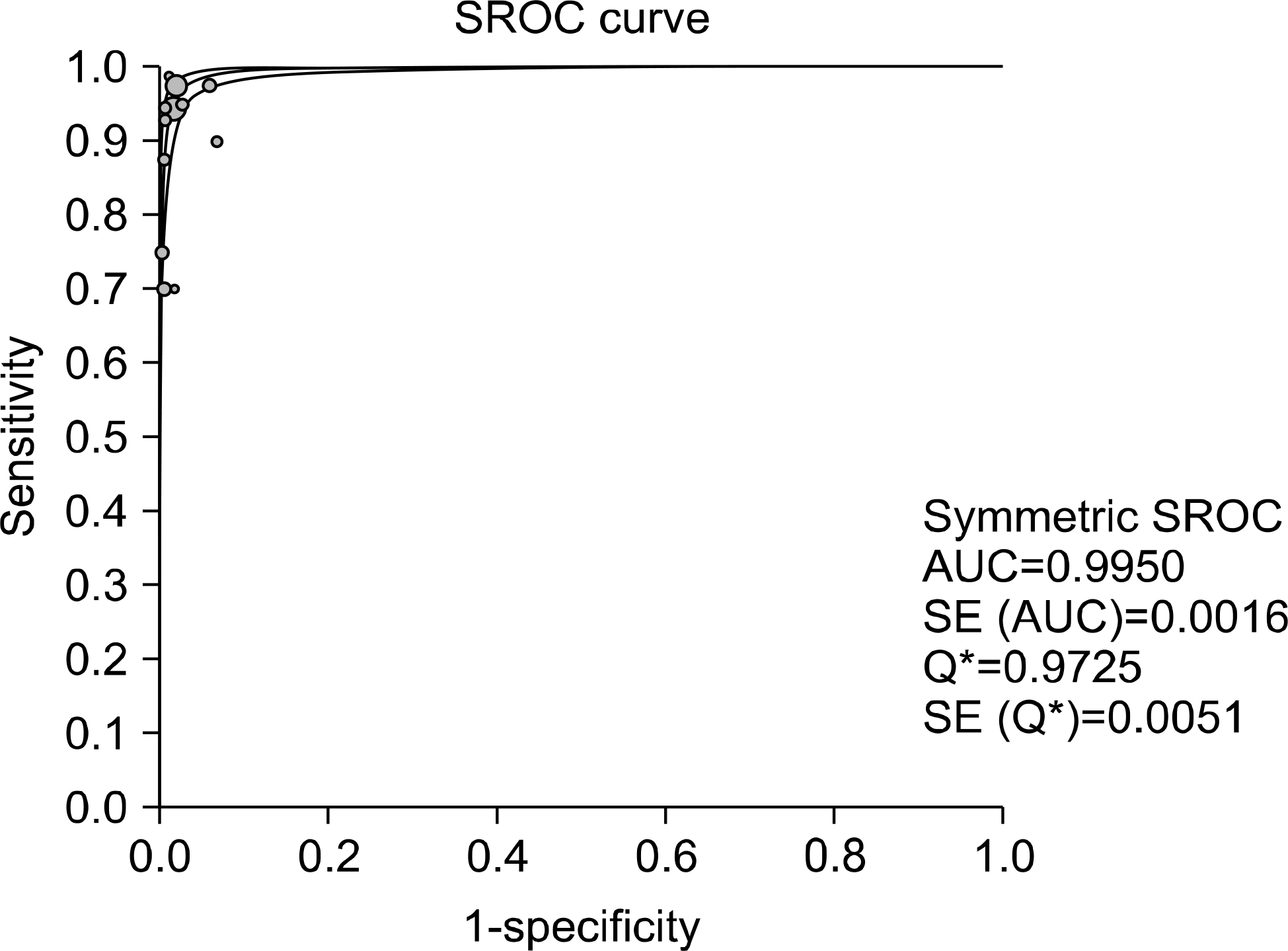
Test accuracy for the detection of M. tuberculosis was assessed on the basis of 20 studies using the M. tuberculosis culture test as the reference standard. The acid-fast bacteria smearing test had a sensitivity in the range of 0.69-1.00, specificity in the range of 0.72-1.00 and test accuracy in the range of 0.75-1.00. Test accuracy regarding rifampin resistance was assessed on the basis of 17 studies. Using an antituberculosis agent sensitivity test as the reference standard, the sensitivity, specificity and test accuracy of real-time, nested PCR were in the ranges of 0.75-1.00, 0.96-1.00 and 0.95-1.00, respe-ctively.
Go to : 
REFERENCES
1.Chang HE., Heo SR., Yoo KC., Song SH., Kim SH., Kim HB, et al. Detection of Mycobacterium tuberculosis complex using real-time polymerase chain reaction. Korean J Lab Med. 2008. 28:103–8.
2.WHO. WHO web sites on infectious diseases. Global Tuberculosis Report 2013.http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Online] (last visited on 25 April 2014).
3.Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 2012. 89–90.
4.Bowles EC., Freyée B., van Ingen J., Mulder B., Boeree MJ., van Soolingen D. Xpert MTB/RIF®, a novel automated polymerase chain reaction-based tool for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2011. 15:988–9.
5.Causse M., Ruiz P., Gutiérrez-Aroca JB., Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol. 2011. 49:3065–7.

6.Blakemore R., Story E., Helb D., Kop J., Banada P., Owens MR, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010. 48:2495–501.

7.Scott LE., McCarthy K., Gous N., Nduna M., Van Rie A., Sanne I, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med. 2011. 8:e1001061.

8.Boehme CC., Nabeta P., Hillemann D., Nicol MP., Shenai S., Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010. 363:1005–15.

9.Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developer's handbook. http://www.sign.ac.uk/guidelines/fulltext/50/[Online. (last visited on 25 April 2014).
10.Boehme CC., Nicol MP., Nabeta P., Michael JS., Gotuzzo E., Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011. 377:1495–505.

11.Friedrich SO., Venter A., Kayigire XA., Dawson R., Donald PR., Diacon AH. Suitability of Xpert MTB/RIF and genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J Clin Microbiol. 2011. 49:2827–31.

12.Ioannidis P., Papaventsis D., Karabela S., Nikolaou S., Panagi M., Raftopoulou E, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011. 49:3068–70.
13.Lawn SD., Brooks SV., Kranzer K., Nicol MP., Whitelaw A., Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011. 8:e1001067.

14.Malbruny B., Le Marrec G., Courageux K., Leclercq R., Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and nonrespiratory samples. Int J Tuberc Lung Dis. 2011. 15:553–5.
15.Rachow A., Zumla A., Heinrich N., Rojas-Ponce G., Mtafya B., Reither K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay--a clinical validation study. PLoS One. 2011. 6:e20458.
16.Theron G., Peter J., van Zyl-Smit R., Mishra H., Streicher E., Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011. 184:132–40.

17.Vadwai V., Boehme C., Nabeta P., Shetty A., Alland D., Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011. 49:2540–5.

18.Helb D., Jones M., Story E., Boehme C., Wallace E., Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010. 48:229–37.
19.Armand S., Vanhuls P., Delcroix G., Courcol R., Lemaître N. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011. 49:1772–6.
20.Hillemann D., Rüsch-Gerdes S., Boehme C., Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011. 49:1202–5.

21.Marlowe EM., Novak-Weekley SM., Cumpio J., Sharp SE., Momeny MA., Babst A, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011. 49:1621–3.
22.Miller MB., Popowitch EB., Backlund MG., Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011. 49:3458–62.
23.Moure R., Muñoz L., Torres M., Santin M., Martín R., Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011. 49:1137–9.
24.Teo J., Jureen R., Chiang D., Chan D., Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium tuberculosis Direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011. 49:3659–62.
25.Steingart KR., Schiller I., Horne DJ., Pai M., Boehme CC., Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014. 1:CD009593.

26.Chang K., Lu W., Wang J., Zhang K., Jia S., Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012. 64:580–8.
27.Park JS. Recent advances in tuberculosis and nontuberculous mycobacteria lung disease. Tuberc Respir Dis. 2013. 74:251–5.

28.Kwak N., Choi SM., Lee J., Park YS., Lee CH., Lee SM, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013. 8:e77456.

29.Lee HY., Seong MW., Park SS., Hwang SS., Lee J., Park YS, et al. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013. 17:917–21.

Go to : 
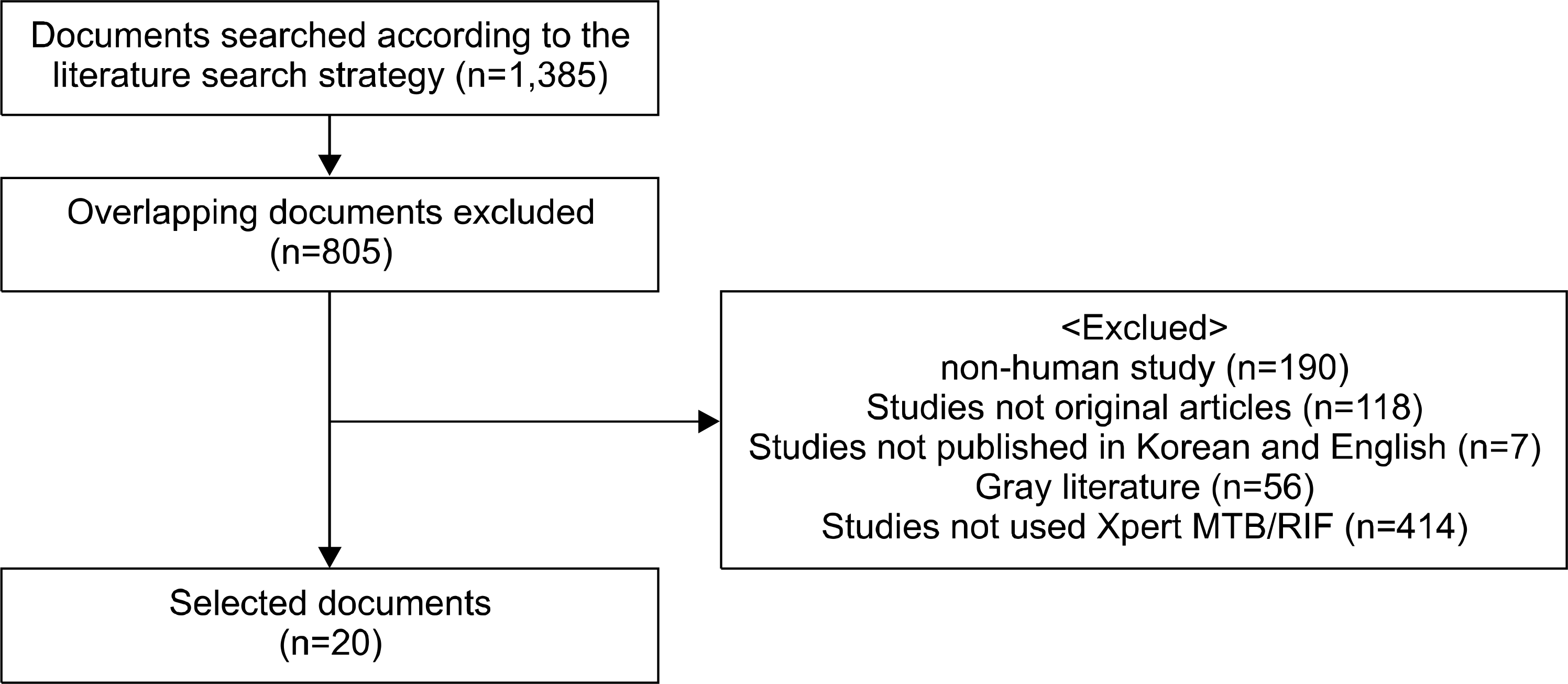 | Fig. 1.Documents selected for evaluation of Xpert MTB/RIF according to the literature search strategy. |
Table 1.
Levels of evidence [9]
Table 2.
Grades of recommendations [9]
Table 3.
Documents selected for evaluation of Xpert MTB/RIF
| No | Author (Publication year) | Research location Insi | No. of cases | |
|---|---|---|---|---|
| inside lung sample | Outside lung sample | |||
| 1 | Armand (2011) [19]∗ | France | 60 | 37 |
| 2 | Boehme (2011) [10] | South Africa, Peru, India, Azerbaijan, Philippines, Uganda | 3,909 | - |
| 3 | Bowles (2011) [4]∗ | Netherlands | 87 | 2 |
| 4† | Causse (2011) [5]∗ | Spain | - | 340 |
| 5 | Friedrich (2011) [11] | South Africa | 140 | - |
| 6 | Hillemann (2011) [20]∗ | Germany | - | 521 |
| 7 | Ioannis (2011) [12] | Greece | 80 | 41 |
| 8 | Lawn (2011) [13] | South Africa | 839 | - |
| 9 | Malbruny (2011) [14] | France | 91 | 89 |
| 10 | Marlowe (2011) [21]∗ | USA | 217 | - |
| 11† | Miller (2011) [22]∗ | USA | 89 | 23 |
| 12 | Moure (2011) [23]∗ | Spain | 105 | 20 |
| 13 | Rachow (2011) [15] | Tanzania | 172 | - |
| 14 | Scott (2011) [7]∗ | South Africa | 177 | - |
| 15† | Teo (2011) [24]∗ | Singapore | 131 | 31 |
| 16 | Theron (2011) [16] | South Africa | 480 | - |
| 17 | Vadwai (2011) [17] | India | - | 547 |
| 18 | Blakemore (2010) [6]∗ | USA | 168 | - |
| 19 | Boehme (2010) [8] | Peru, Azerbaijan, South Africa, India | 1,462 | - |
| 20 | Helb (2010) [18] | Vietnam, Uganda | 107 | 64 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download