초록
The Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) have recently revised the susceptibility interpretive criteria of oxyimino-β -lactams and carbapenems for Enterobacteriaceae. According to the new criteria, susceptibility testing results are sufficient to detect extended-spectrum β -lactamases (ESBLs) and carbapenemases; it is not necessary to perform ESBL or carbapenemase detection tests for therapeutic purposes. Thus, it has been recomme-nded that these related tests be performed only for infection control. These changes in the susceptibility guidelines are supported by some clinical cases and the results of pharmacodynamic and animal studies. However, differences still exist between the breakpoints established by the CLSI and EUCAST with re-gard to some oxyimino-β -lactam and carbapenem an-tibiotics, in particular, the breakpoints for ceftazidime and cefepime established by the CLSI are higher than those established by the EUCAST. Also, similar num-bers of successful and unsuccessful cases have been reported regarding the use of cephalosporins or carbapenems in treating infections caused by low-mini-mal inhibitory concentration (MIC) ESBL-producers or low-MIC carbapenemase-producers. Finally, routine susceptibility test methods are not as accurate as re-search-purpose test methods, showing differences in MICs ranging approximately from 1 to 8 μ g/mL. In conclusion, it is strategically prudent to continue to perform ESBL and carbapenemase detection tests and to avoid the use of the corresponding antimicrobial agents for the treatment of ESBL or carbapene-mase-producing bacterial infections.
Go to : 
REFERENCES
1.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement. Document M100-S19. Wayne, PA; Clinical and Laboratory Standards Institute. 2009.
2.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement. Document M100-S21. Wayne, PA; Clinical and Laboratory Standards Institute. 2011.
3.Leclercq R., Cantón R., Brown DF., Giske CG., Heisig P., MacGowan AP, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013. 19:141–60.

4.Thomson KS., Sanders CC. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob Agents Chemother. 1992. 36:1877–82.
5.Coudron PE. Inhibitor-based methods for detection of plasmid- mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J Clin Microbiol. 2005. 43:4163–7.
6.Song W., Jeong SH., Kim JS., Kim HS., Shin DH., Roh KH, et al. Use of boronic acid disk methods to detect the combined expression of plasmid-mediated AmpC beta-lactamases and extended- spectrum beta-lactamases in clinical isolates of Klebsiella spp., Salmonella spp., and Proteus mirabilis. Diagn Microbiol Infect Dis. 2007. 57:315–8.
7.Song W., Bae IK., Lee YN., Lee CH., Lee SH., Jeong SH. Detection of extended-spectrum beta-lactamases by using boronic acid as an AmpC beta-lactamase inhibitor in clinical isolates of Klebsiella spp. and Escherichia coli. J Clin Microbiol. 2007. 45:1180–4.
8.Jeong SH., Song W., Park MJ., Kim JS., Kim HS., Bae IK, et al. Boronic acid disk tests for identification of extended-spectrum beta-lactamase production in clinical isolates of Enterobacteriaceae producing chromosomal AmpC beta-lactamases. Int J Antimicrob Agents. 2008. 31:467–71.
9.Lee K., Kim MN., Kim JS., Hong HL., Kang JO., Shin JH, et al. KONSAR Group. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011. 52:793–802.
10.Queenan AM., Bush K. Carbapenemases: the versatile beta- lactamases. Clin Microbiol Rev. 2007. 20:440–58.
11.Nordmann P., Cuzon G., Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009. 9:228–36.
12.Rhee JY., Park YK., Shin JY., Choi JY., Lee MY., Peck KR, et al. KPC-producing extreme drug-resistant Klebsiella pneumoniae isolate from a patient with diabetes mellitus and chronic renal failure on hemodialysis in South Korea. Antimicrob Agents Chemother. 2010. 54:2278–9.
13.Roh KH., Lee CK., Sohn JW., Song W., Yong D., Lee K. Isolation of a Klebsiella pneumoniae isolate of sequence type 258 producing KPC-2 carbapenemase in Korea. Korean J Lab Med. 2011. 31:298–301.
14.Walsh TR., Toleman MA., Poirel L., Nordmann P. Metallo-beta- lactamases: the quiet before the storm? Clin Microbiol Rev. 2005. 18:306–25.
15.Ito H., Arakawa Y., Ohsuka S., Wacharotayankun R., Kato N., Ohta M. Plasmid-mediated dissemination of the metallo-beta-lactamase gene bla IMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995. 39:824–9.
16.Lee K., Yum JH., Yong D., Lee HM., Kim HD., Docquier JD, et al. Novel acquired metallo-beta-lactamase gene, bla SIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005. 49:4485–91.
17.Poirel L., Potron A., Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012. 67:1597–606.

18.Song W., Suh B., Choi JY., Jeong SH., Jeon EH., Lee YK, et al. In vivo selection of carbapenem-resistant Klebsiella pneumoniae by OmpK36 loss during meropenem treatment. Diagn Microbiol Infect Dis. 2009. 65:447–9.
19.Shin SY., Bae IK., Kim J., Jeong SH., Yong D., Kim JM, et al. Resistance to carbapenems in sequence type 11 Klebsiella pneumoniae is related to DHA-1 and loss of OmpK35 and/or OmpK36. J Med Microbiol. 2012. 61:239–45.
20.Park YJ., Yu JK., Park KG., Park YG., Lee S., Kim SY, et al. Prevalence and contributing factors of nonsusceptibility to imipenem or meropenem in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Diagn Microbiol Infect Dis. 2011. 71:87–9.
21.Cohen Stuart J., Leverstein-Van Hall MA. Dutch Working Party on the Detection of Highly Resistant Microorganisms. Guideline for phenotypic screening and confirmation of carbapenemases in Enterobacteriaceae. Int J Antimicrob Agents. 2010. 36:205–10.
22.Girlich D., Poirel L., Nordmann P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol. 2012. 50:477–9.
23.Nordmann P., Poirel L., Dortet L. Rapid detection of carbapenemase- producing Enterobacteriaceae. Emerg Infect Dis. 2012. 18:1503–7.
24.Dortet L., Poirel L., Nordmann P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother. 2012. 56:6437–40.
25.Giske CG., Gezelius L., Samuelsen Ø., Warner M., Sundsfjord A., Woodford N. A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with amino-phenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011. 17:552–6.
26.Tsakris A., Themeli-Digalaki K., Poulou A., Vrioni G., Voulgari E., Koumaki V, et al. Comparative evaluation of combined-disk tests using different boronic acid compounds for detection of klebsiella pneumoniae carbapenemase-producing enterobacteriaceae clinical isolates. J Clin Microbiol. 2011. 49:2804–9.
27.European Committee on Antimicrobial Susceptibility Testing. EUCAST clinical breakpoint v 3.1. http://www.eucast.org/clinical_breakpoints/. [Online] (last visited on 1 June 2013).
28.Andes D., Craig WA. Treatment of infections with ESBL-pro-ducing organisms: pharmacokinetic and pharmacodynamic consi-derations. Clin Microbiol Infect. 2005. 11(Suppl 6):10–7.

29.Maglio D., Ong C., Banevicius MA., Geng Q., Nightingale CH., Nicolau DP. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob Agents Chemother. 2004. 48:1941–7.
30.Paterson DL., Ko WC., Von Gottberg A., Casellas JM., Mulazimoglu L., Klugman KP, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001. 39:2206–12.
31.Daikos GL., Petrikkos P., Psichogiou M., Kosmidis C., Vryonis E., Skoutelis A, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2009. 53:1868–73.
32.Livermore DM., Andrews JM., Hawkey PM., Ho PL., Keness Y., Doi Y, et al. Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother. 2012. 67:1569–77.

33.Katsanis GP., Spargo J., Ferraro MJ., Sutton L., Jacoby GA. Detection of Klebsiella pneumoniae and Escherichia coli strains producing extended-spectrum beta-lactamases. J Clin Microbiol. 1994. 32:691–6.
34.Song W., Park MJ., Kim HS., Kim JS., Kim HS., Lee KM. Comparison of Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing breakpoints for beta-lactams in Enterobacteriaceae producing extended-spectrum beta-lactamases and/or plasmid-mediated Ampc beta-lactamases. Korean J Clin Microbiol. 2011. 14:24–9.
35.Bin C., Hui W., Renyuan Z., Yongzhong N., Xiuli X., Yingchun X, et al. Outcome of cephalosporin treatment of bacteremia due to CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli. Diagn Microbiol Infect Dis. 2006. 56:351–7.
36.Ho PL., Chan WM., Tsang KW., Wong SS., Young K. Bacteremia caused by Escherichia coli producing extended-spectrum beta- lactamase: a case-control study of risk factors and outcomes. Scand J Infect Dis. 2002. 34:567–73.
37.Weisenberg SA., Morgan DJ., Espinal-Witter R., Larone DH. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn Microbiol Infect Dis. 2009. 64:233–5.
38.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aero-bically; Approved Standard Seventh Edition. Document M7-A7. Wayne, PA; Clinical and Laboratory Standards Institute. 2006.
39.Lat A., Clock SA., Wu F., Whittier S., Della-Latta P., Fauntleroy K, et al. Comparison of polymyxin B, tigecycline, cefepime, and meropenem MICs for KPC-producing Klebsiella pneumoniae by broth microdilution, Vitek 2, and Etest. J Clin Microbiol. 2011. 49:1795–8.
40.Giakkoupi P., Tzouvelekis LS., Daikos GL., Miriagou V., Petrikkos G., Legakis NJ, et al. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J Clin Microbiol. 2005. 43:494–6.
41.Woodford N., Turton JF., Livermore DM. Multiresistant gram- negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011. 35:736–55.
42.Kitchel B., Rasheed JK., Patel JB., Srinivasan A., Navon-Venezia S., Carmeli Y, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009. 53:3365–70.
43.Paul M., Shani V., Muchtar E., Kariv G., Robenshtok E., Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010. 54:4851–63.

44.Schwaber MJ., Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007. 60:913–20.
Go to : 
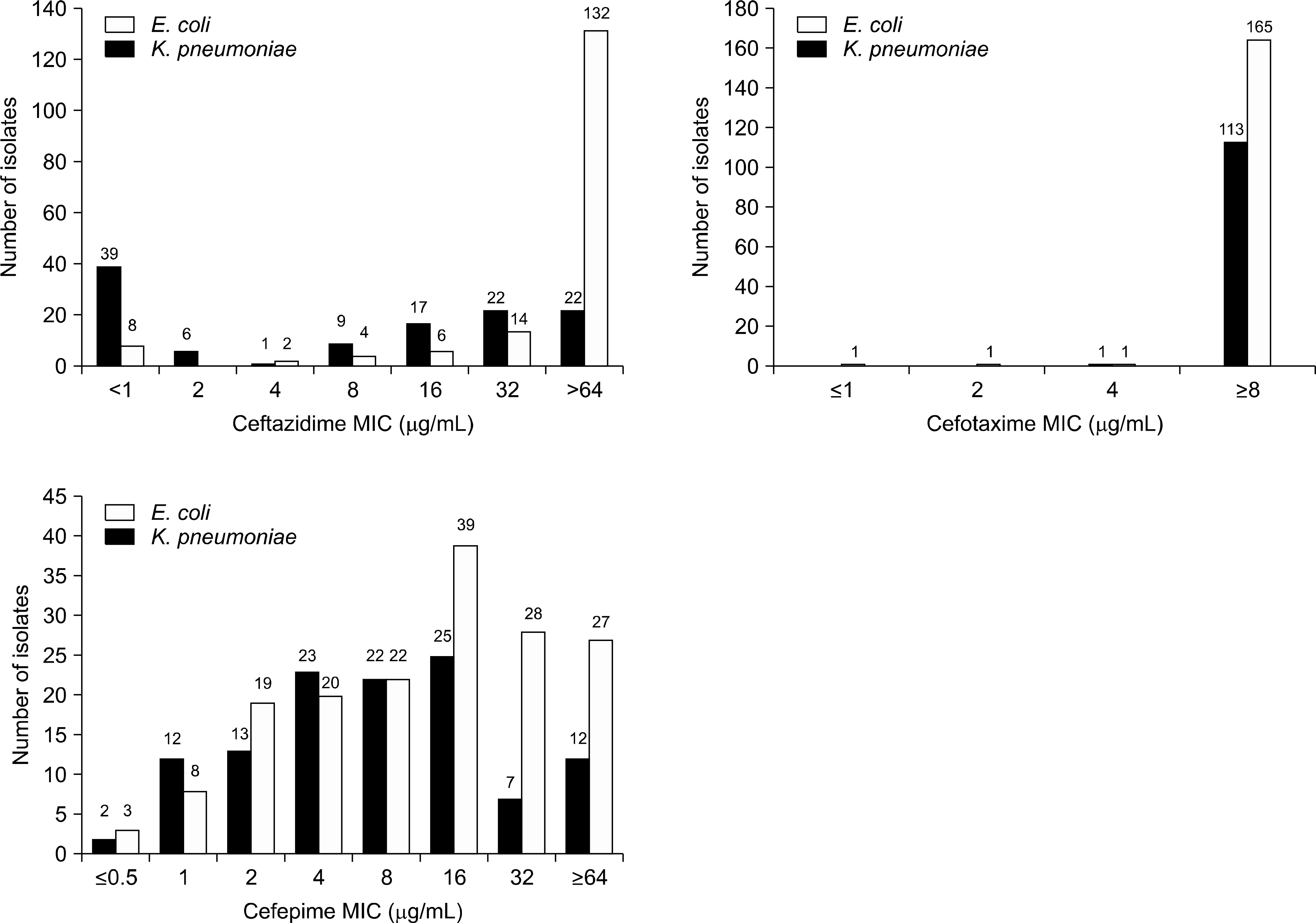 | Fig. 1.Distribution of MICs by new CLSI interpretation criteria for E. coli and K. pneumoniae isolates producing ESBLs in Korea. |
Table 1.
Interpretation of carbapenemase phenotypic tests
Table 2.
Old and new interpretative standards for Enterobacteriaceae




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download