초록
Background
Methods
Results
Conclusion
REFERENCES
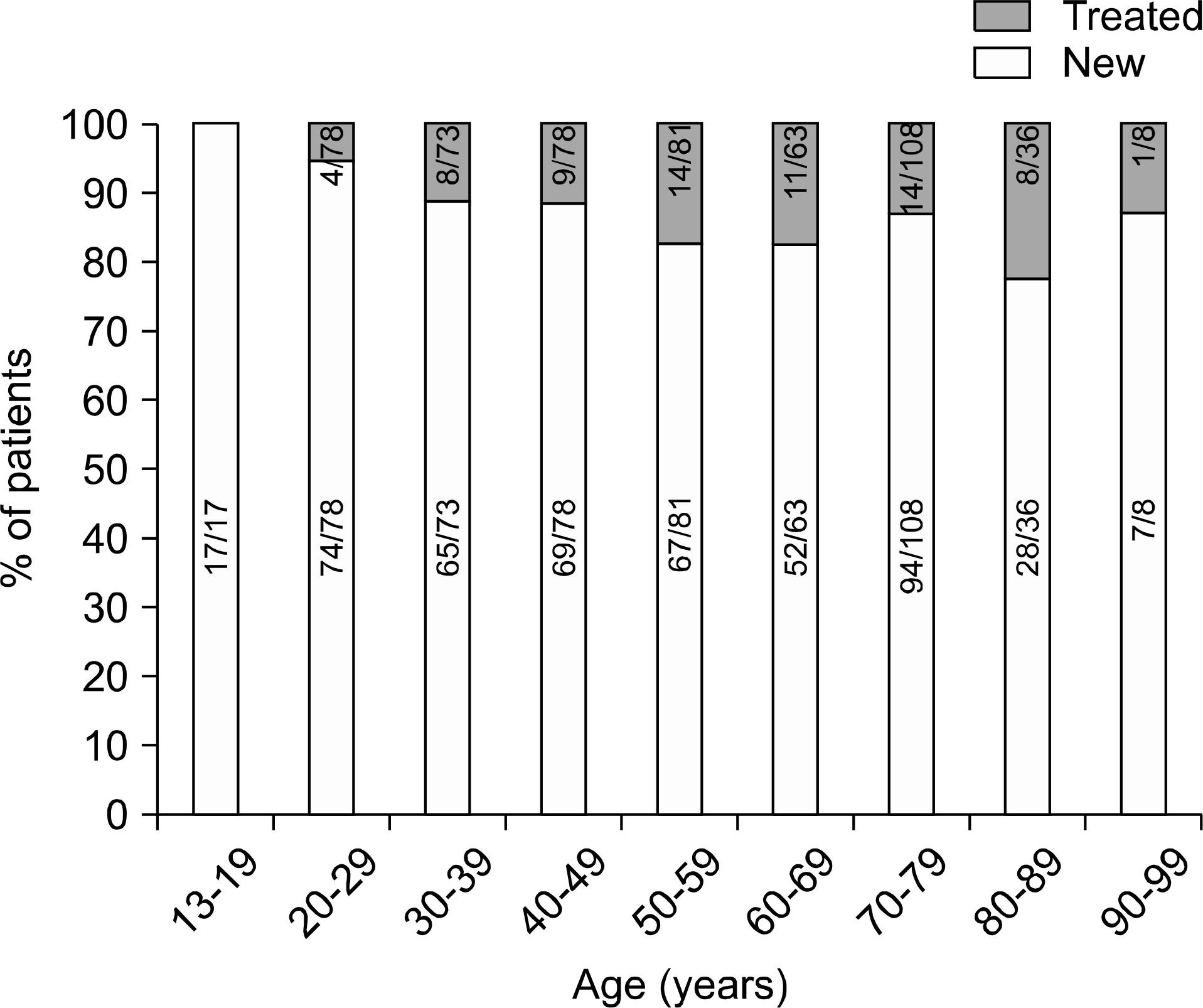 | Fig. 1.Age distribution among newly and previously treated patients in this study. Abbreviations: New, newly treated patient; Treated, previously treated patient. |
Table 1.
| No (%) | Total no (% of patients) | P value | ||
|---|---|---|---|---|
| New (%) | Treated (%) | |||
| Total tested | 542 | 473 | 69 | |
| ≥ One∗ | 98 (18.1) | 69 (14.6) | 29 (42.0) | <0.001 |
| Resistance to | ||||
| RFP | 39 (7.2) | 21 (4.4) | 18 (26.1) | <0.001 |
| INH | 74 (13.7) | 49 (10.4) | 25 (36.2) | <0.001 |
| EMB | 25 (4.6) | 13 (2.7) | 12 (17.4) | <0.001 |
| PZA | 21 (3.9) | 11 (2.3) | 10 (14.5) | <0.001 |
| RBT | 29 (5.4) | 16 (3.4) | 13 (18.8) | 0.002 |
| SM | 36 (6.6) | 26 (5.5) | 10 (14.5) | <0.001 |
| AMK | 5 (0.9) | 1 (0.2) | 4 (5.8) | <0.001 |
| KM | 5 (0.9) | 1 (0.2) | 4 (5.8) | 0.002 |
| CPM | 4 (0.7) | 1 (0.2) | 3 (4.3) | 0.006 |
| OFX | 19 (3.5) | 7 (1.5) | 12 (17.4) | <0.001 |
| LEV | 19 (3.5) | 7 (1.5) | 12 (17.4) | <0.001 |
| MXF | 14 (2.6) | 5 (1.1) | 9 (13.0) | <0.001 |
| PTH | 15 (2.8) | 10 (2.1) | 5 (7.2) | 0.010 |
| CS | 3 (0.6) | 1 (0.2) | 2 (2.9) | <0.001 |
| PAS | 17 (3.1) | 12 (2.5) | 5 (7.2) | <0.001 |
| MDR-TB | 33 (6.1) | 18 (3.8) | 15 (21.7) | 0.001 |
| XDR-TB | 3 (0.6) | 0 | 3 (4.3) | 0.001 |
∗ Resistant to one or more antituberculosis drugs among 15 anti- tuberculosis drugs using in this study.
Abbreviations: New, newly treated patient; Treated, previously treated patient; RFP, rifampin; INH, isoniazid; EMB, ethambutol; PZA, pyrazinamide; RBT, rifabutin; SM, streptomycin; AMK, amikacin; KM, kanamycin; CPM, capreomycin; OFX, ofloxacin; LEV, levofloxacin; MXF, moxifloxacin; PTH, prothionamide; CS, cycloserine; PAS, para-aminosalicylic acid; MDR-TB, multi-drug resistance TB including rifampin and isoniazid resistance; XDR-TB, extensively drug resistance TB.
Table 2.
∗ Resistant to one or more antituberculosis drugs among 15 antituberculosis drugs using in this study.
Abbreviations: New, newly treated patient; Treated, previously treated patient; RFP, rifampin; INH, isoniazid; EMB, ethambutol; PZA, pyrazinamide; RBT, rifabutin; MDR-TB, multi-drug resistance TB including rifampin and isoniazid resistance; SMC, Samsung Medical Center; CMCS, Seoul St. Mary's Hospital, The Catholic University of Korea; EWUH, Ewha Womans University Mokdong Hospital; CMCD, Daejeon St. Mary's Hospital, The Catholic University of Korea; PNUH, Pusan National University Yangsan Hospital; CNUH, Chonnam National University Hospital.
Table 3.
Abbreviations: New, newly treated patient; Treated, previously treated patient; SM, streptomycin; KM, kanamycin; AMK, amikacin; CPM, capreomycin; OFX, ofloxacin; LEV, levofloxacin; MXF, moxifloxacin; PTH, prothionamide; CS, cycloserine; PAS, para-aminosalicylic acid; SMC, Samsung Medical Center; CMCS, Seoul St. Mary's Hospital, The Catholic University of Korea; EWUH, Ewha Womans University Mokdong Hospital; CMCD, Daejeon St. Mary's Hospital, The Catholic University of Korea; PNUH, Pusan National University Yangsan Hospital; CNUH, Chonnam National University Hospital.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download