This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
We evaluated the learning curve and short-term surgical outcomes of robot-assisted distal gastrectomy (RADG) performed by a single surgeon experienced in open, but not laparoscopic, gastrectomy. We aimed to verify the feasibility of performing RADG without extensive laparoscopic experience.
Materials and Methods
Between July 2012 and December 2016, 60 RADG procedures were performed by a single surgeon using the da Vinci® Surgical System (Intuitive Surgical). Patient characteristics, the length of the learning curve, surgical parameters, and short-term postoperative outcomes were analyzed and compared before and after the learning curve had been overcome.
Results
The duration of surgery rapidly decreased from the first to the fourth case; after 25 procedures, the duration of surgery was stabilized, suggesting that the learning curve had been overcome. Cases were divided into 2 groups: 25 cases before the learning curve had been overcome (early cases) and 35 later cases. The mean duration of surgery was 420.8 minutes for the initial cases and 281.7 minutes for the later cases (P<0.001). The console time was significantly shorter during the later cases (168.6 minutes) than during the early cases (247.1 minutes) (P<0.001). Although the volume of blood loss during surgery declined over time, there was no significant difference between the early and later cases. No other postoperative outcomes differed between the 2 groups. Pathology reports revealed the presence of mucosal invasion in 58 patients and submucosal invasion in 2 patients.
Conclusions
RADG can be performed safely with acceptable surgical outcomes by experts in open gastrectomy.
Keywords: Robotic surgical procedures, Stomach neoplasms, Learning curve, Gastrectomy
INTRODUCTION
Due to advanced robotic technologies, including superior 3-dimensional views, improved dexterity using an internally articulated EndoWrist
® (Intuitive Surgical, Sunnyvale, CA, USA), increased stability, and superior ergonomics compared with conventional laparoscopy [
1], robotic surgery has been rapidly applied in general surgery [
234]. Robotic systems enable surgeons to control a camera and robotic arms so they can perform complicated procedures more easily and precisely [
5].
Gastric cancer is the second-most common cancer in Korea, and the country's third-most common cause of cancer death [
6]. The number of patients with early gastric cancer has consistently increased, accounting for 61.0% of all cases of gastric cancer in 2014 [
7]. More than 50% of gastrectomies are performed using a minimally invasive approach, which has increased in popularity [
7]. Several retrospective gastrectomy studies have reported less blood loss during surgery, shorter hospital stays, and easier lymph node (LN) retrieval with the use of robotic gastrectomy compared to laparoscopic gastrectomy [
89101112]; however, some meta-analyses and prospective studies of robotic gastrectomy did not show significant differences in postoperative outcomes between robotic and laparoscopic gastrectomies [
1314151617]. The cumulative experience of surgeons has been shown to be more important than the type of mechanical device used, because most robotic surgeries are performed by experienced laparoscopic surgeons. In addition, it is likely that most laparoscopic or robotic surgeries are performed by younger surgeons; older surgeons tend to prefer the more familiar open surgeries over the more minimally invasive approaches used in robotic surgeries.
Although robotic surgery is usually performed by experienced laparoscopic surgeons, the advantage of robotic systems may help surgeons who are more experienced with open surgery. The articulated function and delicate robotic movements may make EndoWrist® more convenient and easier to use than typical laparoscopic instruments. At present, most data gathered during robotic gastrectomies are extracted from procedures performed by experienced laparoscopic surgeons. Scant data have been collected from surgeons accustomed to performing open gastrectomy who recently began performing robotic surgery. Considering the devices used in robotic systems, transitioning from open gastrectomy to robotic gastrectomy may be possible, even without extensive laparoscopic experience.
Therefore, we evaluated the learning curve of a single surgeon with extensive experience in open gastrectomy, but with limited experience in laparoscopic gastrectomy, over 60 robot-assisted distal gastrectomy (RADG) procedures. We also assessed the short-term surgical outcomes of these cases.
MATERIALS AND METHODS
Patients
We analyzed prospectively collected data from 60 consecutive patients who underwent RADG performed by a single surgeon between July 2012 and December 2016 at our institution. Since the surgeon performed approximately 800 open gastrectomies and only 30 laparoscopy-assisted distal gastrectomies during the same period, as well as over 6,000 open gastrectomies during the course of his career, we considered him as with extensive open surgery experience. The indication for RADG was confined to early stage gastric cancer diagnosed by preoperative esophagogastroduodenoscopy and/or abdominal computed tomography. All data were collected prospectively, which began with the surgeon's first RADG case. This study was approved by the Institutional Review Board of Samsung Medical Center (2016-10-109).
Surgical procedure
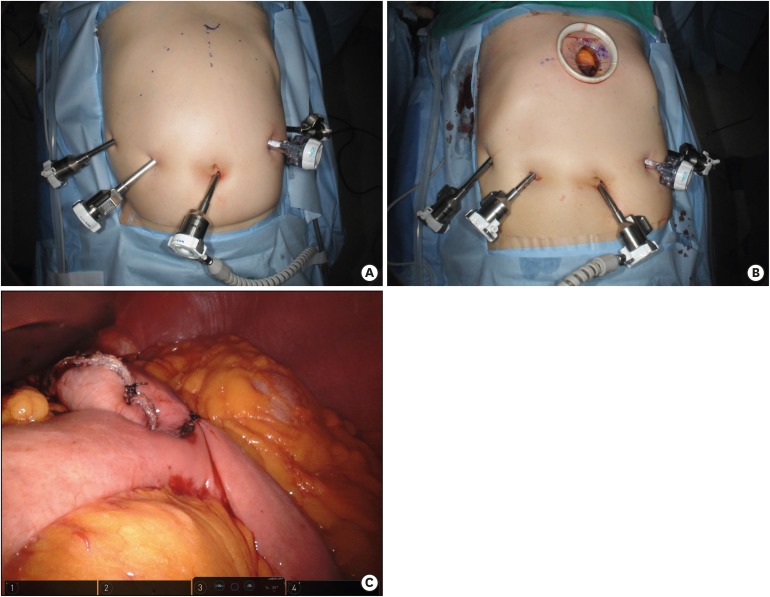
After the camera port is inserted below the umbilicus with a 12-mm trocar, 4 ports (one 12-mm and three 8-mm in diameter) are placed under camera visualization (
Fig. 1A). The patient is placed in the reverse Trendelenburg position and docking of the robotic arms is performed. Then, the surgeon starts partial omentectomy toward the lower pole of the spleen with ultrasonic shears on the console. After dividing left and right gastroepiploic and right gastric arteries, the duodenum is transected 1–2 cm distal to the pyloric ring using an endoscopic linear stapler. In the suprapancreatic area, LNs around the proper and common hepatic artery are dissected. With exposure and ligation of the left gastric vein and artery, LNs in this area are also dissected. The retroperitoneal attachment of the stomach is detached up to the right diaphragmatic crus. Perigastric LNs along the lesser curvature of the stomach are dissected. After complete mobilization of the stomach, un-docking of the robotic arms is performed for extracorporeal anastomosis. Through a 4–5-cm mini-laparotomy incision in the epigastrium, the mobilized stomach is extracted and tumor location is confirmed by palpation of clips that were applied a day before surgery by an endoscopist (
Fig. 1B). After gastric resection using two 60-mm endo-linear staplers, gastrojejunostomy is performed using a 60-mm endo-linear stapler. The common entry hole for the anastomosis is closed with a 60-mm endo-linear stapler or hand-sewing. In this study, all patients received gastrojejunostomy (
Fig. 1C).
Fig. 1
Operative procedures of robot-assisted distal gastrectomy. (A) Port placements. (B) Mini-laparotomy for gastric resection and anastomosis. (C) Completion of distal gastrectomy and gastrojejunostomy.
Learning curve analysis
To assess the learning curve for RADG, we used the exponential weighted moving average (EWMA) method developed by Roberts [
18]. The EWMA method uses a moving average for current and historical observations with weights that decay exponentially. The EWMA for a series of surgical durations
Tn for
n = 1, ..., 60 is computed as:
where
EWMA0 is the mean of historical data, λ is a constant smoothing parameter, such that 0 < λ < 1,
Tn is the
nth duration of surgery, and
EWMAn is the value of the EWMA of the
nth surgery. The smoothing parameter λ is usually set between 0.05 and 0.25 [
19]. The upper and lower control limits are:
where
l is typically set to 3 [
20]; it is necessary to reduce
l slightly for small values of λ [
19].
Analysis of postoperative outcomes
After assessing the learning curve using the EWMA method, patients were divided into 2 groups: early cases, those performed before the learning curve was overcome, and later cases, those performed after the learning curve was overcome. The characteristics of the enrolled patients, including age, sex, American Society of Anesthesiologists (ASA) score, history of any previous abdominal surgery, body mass index (BMI), and tumor classification, were reviewed and compared between the 2 groups. Factors associated with perioperative outcomes, such as the presence of combined resection, duration of surgery, console time, volume of blood loss during surgery, length of hospital stay, time to first flatus, and time to soft diet initiation, were reviewed.
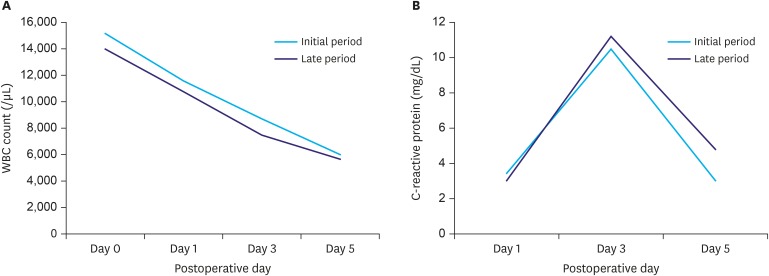
To evaluate inflammatory responses, white blood cell (WBC) counts were obtained immediately after surgery and on postoperative days (PODs) 1, 3, and 5; a serum C-reactive protein (CRP) level was obtained on PODs 1, 3, and 5. Surgical complications were recorded according to the Clavien-Dindo classification.
Statistical methods
SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze the demographics and perioperative outcomes of patients in each group. Pearson's χ2 test was used to compare categorical variables. An independent t-test was used for continuous variables if the data for both groups satisfied the criteria for normality; otherwise, the Mann-Whitney U test was used. To compare longitudinal outcomes, such as WBC counts and serum CRP levels, a linear mixed model was applied, and the outcomes at each time point were compared with independent t-tests. To describe the learning curve, the point at which the duration of surgery stabilized was evaluated against the EWMA chart, using the package “qcc” in R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). P-values less than 0.05 were considered statistically significant.
RESULTS
Learning curve
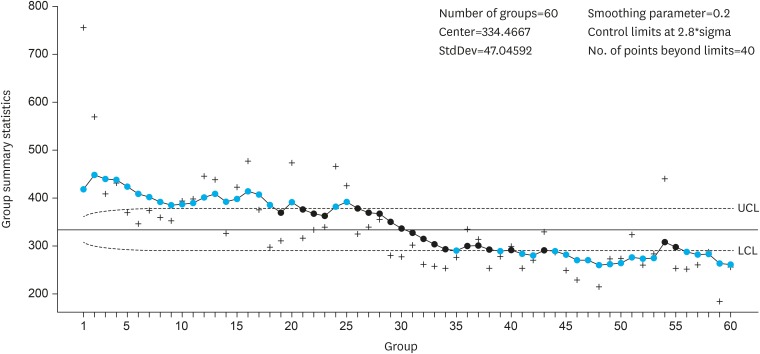
As described above, the duration of surgery was evaluated using the EWMA method with parameters λ=0.2 and
l=2.8, which matched the EWMA chart from a surgeon with more experience in RADG. As shown in
Fig. 2, the duration of surgery (EWMA
n) for 3 RADG cases decreased gradually; the duration of surgery was stabilized after 25 cases, since all EWMA
n values were under the upper control limit. Some EWMA
n values were slightly under the lower control limit after 35 cases.
Fig. 2
Learning curve analysis using the EWMA method.
EWMA = exponential weighted moving average; UCL = upper control limit; LCL = lower control limit.
The learning curve was overcome after 25 cases.
Patient characteristics
Age, sex, BMI, ASA score, and tumor characteristics were similar between the 2 groups (
Table 1). Since the indication for RADG was early stage gastric cancer, most patients had been diagnosed with stage T1N0 gastric cancer. The mean number of retrieved LNs did not significantly differ between the early and later cases.
Table 1
Patient characteristics
|
Characteristics |
Early cases (n=25) |
Later cases (n=35) |
P-value |
|
Age (yr) |
50.4±9.1 |
52.9±8.5 |
0.283 |
|
Sex |
|
|
1.000 |
|
Male |
14 (56) |
19 (54.3) |
|
Female |
11 (44) |
16 (45.7) |
|
BMI (kg/m2) |
22.5±1.9 |
22.1±2.1 |
0.516 |
|
ASA score |
|
|
0.661 |
|
I |
15 (60.0) |
19 (54.3) |
|
II |
10 (40.0) |
15 (42.9) |
|
III |
0 |
1 (2.9) |
|
Endoscopic clip |
21 (84.0) |
32 (91.4) |
0.436 |
|
Tumor size (cm) |
2.8±1.6 |
2.5±1.6 |
0.565 |
|
Proximal resection margin (cm) |
2.9±1.9 |
3.0±1.4 |
0.790 |
|
Distal resection margin (cm) |
7.2±3.2 |
6.3±2.5 |
0.289 |
|
Depth of tumor invasion |
|
|
0.169 |
|
T1 |
23 (92.0) |
35 (100) |
|
T3 |
2 (8.0) |
0 |
|
LN metastasis |
|
|
0.323 |
|
N0 |
21 (84.0) |
33 (94.3) |
|
N1 |
3 (12.0) |
2 (5.7) |
|
N2 |
1 (4.0) |
0 |
|
Retrieved LN No. |
33.8±10.6 |
36.7±10.4 |
0.298 |
Perioperative outcomes
Perioperative outcomes for each group are shown in
Table 2. All patients underwent distal gastrectomy with gastrojejunostomy and D2 LN dissection. No case was converted to open gastrectomy. The percentage of patients who underwent combined resection was similar in both groups. The duration of surgery and console time were longer in the early group than in the later group (420.8 vs. 281.7 minutes, 247.1 vs. 168.6 minutes, respectively; P<0.001). Although the estimated blood loss volume during surgery was less in the later group, there was no significant difference between groups (214 vs. 91.4 mL; P=0.084). Length of hospital stay, time to first flatus, and time to initiation of a soft diet were similar between groups. Inflammatory markers, such as WBC count and serum CRP level, did not significantly differ between groups (
Fig. 3).
Table 2
Operative and postoperative outcomes
|
Characteristics |
Early cases (n=25) |
Later cases (n=35) |
P-value |
|
D2 LN dissection |
25 (100) |
35 (100) |
1.000 |
|
Combined resection |
2 (8.0) |
2 (5.7) |
1.000 |
|
Open conversion |
0 |
0 |
1.000 |
|
Duration of surgery (min) |
420.8±113.0 |
281.7±45.1 |
<0.001 |
|
Console time (min) |
247.1±67.1 |
168.6±41.8 |
<0.001 |
|
Volume of blood loss during surgery (mL) |
214.0±338.5 |
91.4±44.5 |
0.084 |
|
Hospital stay after surgery (day) |
9.0±4.4 |
8.1±2.2 |
0.297 |
|
Time to first flatus passage (day) |
3.4±0.8 |
3.4±0.6 |
0.931 |
|
Time to initiation of a soft diet (day) |
7.1±4.1 |
5.7±0.9 |
0.727 |
Fig. 3
Postoperative inflammation. (A) WBC counts; (B) CRP levels.
WBC = white blood cell; CRP = C- reactive protein; POD = postoperative day.
Postoperative morbidities
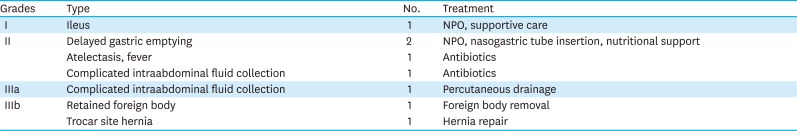
Postoperative morbidities occurred in 9 patients (15%): 6 early cases and 3 later cases. The types of complications are described in
Table 3. One grade IIIa complication developed in a later case, and 2 cases of grade IIIb complications were early cases. There were no cases with anastomotic or stump leakage or surgical site bleeding in either group.
Table 3
Postoperative complications
|
Grades |
Type |
No. |
Treatment |
|
I |
Ileus |
1 |
NPO, supportive care |
|
II |
Delayed gastric emptying |
2 |
NPO, nasogastric tube insertion, nutritional support |
|
Atelectasis, fever |
1 |
Antibiotics |
|
Complicated intraabdominal fluid collection |
1 |
Antibiotics |
|
IIIa |
Complicated intraabdominal fluid collection |
1 |
Percutaneous drainage |
|
IIIb |
Retained foreign body |
1 |
Foreign body removal |
|
Trocar site hernia |
1 |
Hernia repair |
DISCUSSION
This study assessed the robotic gastrectomy learning curve for a surgeon experienced in open, not laparoscopic, surgery. Even though the surgeon performed 30 cases of laparoscopic gastrectomy over 4 years, he was classified as an open gastrectomy-experienced surgeon because he had performed 800 cases of open gastrectomy during the same period and had performed more than 6,000 cases of open gastrectomy over his entire medical career. An analysis of his initial experience performing RADG showed a stabilization of the duration of surgery after 25 cases. Although the duration of surgery significantly decreased after the learning curve was overcome, perioperative outcomes, including patient recovery and surgical complications, were similar in early and late RADG cases performed by this surgeon.
Robotic surgery has been reported to be a safe procedure when performed by laparoscopic surgeons [
21]. A previous report showed that rapid adaptation to robotic surgery from laparoscopic surgery occurred within 10 cases [
10]. Because the surgical procedure for robotic gastrectomy is similar to that of laparoscopic gastrectomy, the rapid adaptation to robotic surgery by laparoscopic surgeons is expected.
However, the limitations of laparoscopic surgery, such as 2-dimensional imaging, restricted range of motion of the instruments, and poor ergonomic positioning, disturbs some surgeons who are experienced in open surgery. This finding may explain why short-term outcomes and robotic gastrectomy learning curves for open gastrectomy-experienced surgeons have not been well studied. Considering the devices used in robotic systems, robotic gastrectomy can be safely performed using basic laparoscopic skills. In some respects, transitioning from open surgery to robotic surgery is easier than transitioning from open surgery to laparoscopic surgery for surgeons experienced in open surgery.
In this study, the short-term outcomes from robotic gastrectomy, including the length of hospital stay, volume of blood loss during surgery, time to first flatus, time to a soft diet initiation and postoperative inflammatory responses, did not differ between the early and late cases. Postoperative complications were acceptable with no major complications reported. Moreover, there was no conversion of any case to open gastrectomy. Surgeons with sufficient experience in open gastrectomy are predicted to be able to perform robotic surgery safely, even during the initial cases when learning the robotic procedure takes place.
In clinical practice, several variables, including age, sex, BMI, history of previous abdominal surgery, type of reconstruction, and extent of LN dissection, may affect the duration of surgery and time to overcome the learning curve for the procedure. Duration of surgery was used to assess the learning curve due to the small number of patients and homogeneity of the surgical technique (the same reconstruction method and the same level of LN dissection were used for all patients). In addition, there was no significant correlation between BMI and duration of surgery. For surgeons with sufficient experience in open gastric cancer surgery, using a robotic system and new surgical devices does not appear to present any difficulties.
In conclusion, the stabilization of the duration of RADG was achieved after 25 cases in a surgeon with extensive experience in open gastrectomy. In addition, RADG was performed successfully and safely with acceptable surgical outcomes during the entire learning curve. It is suggested that RADG is worth attempting for surgeons who are experienced in open gastrectomy and not familiar with laparoscopic approaches.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




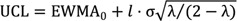
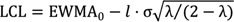


 XML Download
XML Download