1. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20. PMID:
16822992.

2. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730. PMID:
11547741.

3. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321. PMID:
22226517.

4. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID:
17978289.

5. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
6. Park HS, Jung M, Kim HS, Kim HI, An JY, Cheong JH, et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015; 22:224–231. PMID:
25081339.

7. Greenleaf EK, Kulaylat AN, Hollenbeak CS, Almhanna K, Wong J. Timing of adjuvant chemotherapy and impact on survival for resected gastric cancer. Ann Surg Oncol. 2016; 23:4203–4213. PMID:
27459982.

8. Di Bartolomeo M, Pietrantonio F, Rulli E, Poli D, Berenato R, Caporale M, et al. Impact on survival of timing and duration of adjuvant chemotherapy in radically resected gastric cancer. Tumori. 2016; 102:e15–e19. PMID:
27032700.
9. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994; 4:146–148. PMID:
8180768.
10. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010; 251:417–420. PMID:
20160637.
11. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013; 20:1575–1583. PMID:
23076557.

12. Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014; 260:633–638. PMID:
25203880.
13. Malietzis G, Mughal A, Currie AC, Anyamene N, Kennedy RH, Athanasiou T, et al. Factors implicated for delay of adjuvant chemotherapy in colorectal cancer: a meta-analysis of observational studies. Ann Surg Oncol. 2015; 22:3793–3802. PMID:
25777086.

14. Kaito A, Kinoshita T, Shitara K, Shibasaki H, Nishida T. Timing of initiation of adjuvant chemotherapy for gastric cancer: a case-matched comparison study of laparoscopic vs. open surgery. Eur J Surg Oncol. 2017; 43:801–807. PMID:
28187877.

15. Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011; 42:1–28.

16. Colleoni M, Bonetti M, Coates AS, Castiglione-Gertsch M, Gelber RD, Price K, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol. 2000; 18:584–590. PMID:
10653873.
17. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011; 305:2335–2342. PMID:
21642686.
18. Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010; 46:1049–1055. PMID:
20138505.

19. dos Santos LV, Faria TM, Lima AB, Abdalla KC, de Moraes ED, Cruz MR, et al. Timing of adjuvant chemotherapy in colorectal cancer. Colorectal Dis. 2016; 18:871–876. PMID:
26900665.

20. Arrington AK. Timing of chemotherapy and survival in patients with resectable gastric adenocarcinoma. World J Gastrointest Surg. 2013; 5:321–328. PMID:
24392183.

21. Kang SY, Ahn MS, Song GW, Choi YW, Lee HW, Jeong SH, et al. Does the timing of adjuvant chemotherapy for gastric cancer influence patient outcome? Acta Oncol. 2015; 54:1231–1234. PMID:
25608823.

22. Yamamoto M, Sakaguchi Y, Kinjo N, Yamaguchi S, Egashira A, Minami K, et al. S-1 adjuvant chemotherapy earlier after surgery clinically correlates with prognostic factors for advanced gastric cancer. Ann Surg Oncol. 2016; 23:546–551.

23. Fujitani K, Kurokawa Y, Takeno A, Endoh S, Ohmori T, Fujita J, et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer. Forthcoming. 2017.

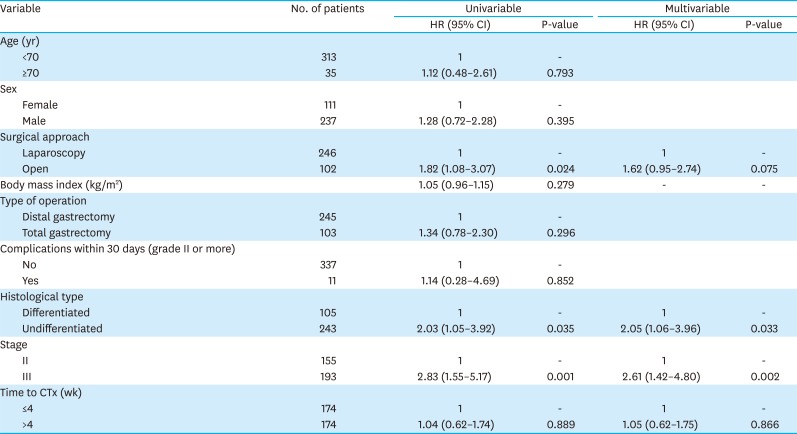
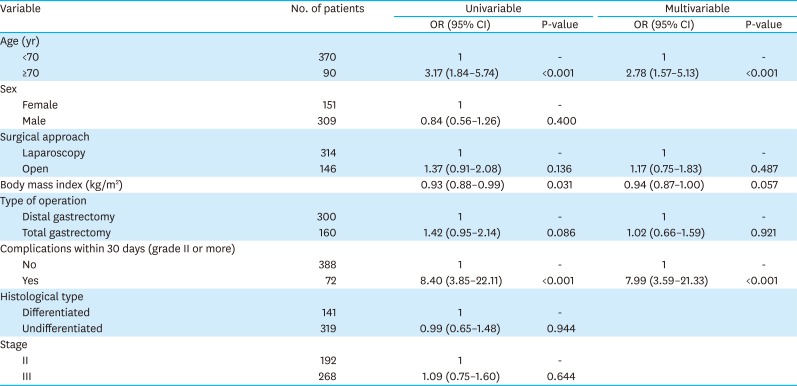




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download