1. Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001; 88:1346–1351. PMID:
11578290.

2. Pross M, Manger T, Reinheckel T, Mirow L, Kunz D, Lippert H. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc. 2000; 51:73–76. PMID:
10625803.

3. Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004; 10:71–75. PMID:
15209546.
4. DaVee T, Irani S, Leggett CL, Berzosa Corella M, Grooteman KV, Wong Kee Song LM, et al. Stent-in-stent technique for removal of embedded partially covered self-expanding metal stents. Surg Endosc. 2016; 30:2332–2341. PMID:
26416379.

5. Langer FB, Schoppmann SF, Prager G, Riegler FM, Zacherl J. Solving the problem of difficult stent removal due to tissue ingrowth in partially uncovered esophageal self-expanding metal stents. Ann Thorac Surg. 2010; 89:1691–1692. PMID:
20417822.

6. Shim CS, Cho YD, Moon JH, Kim JO, Cho JY, Kim YS, et al. Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy. 2001; 33:843–848. PMID:
11571679.

7. Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012; 19:99–103. PMID:
21769467.

8. Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, et al. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011; 73:890–899. PMID:
21521563.

9. Suzuki T, Siddiqui A, Taylor LJ, Cox K, Hasan RA, Laique SN, et al. Clinical outcomes, efficacy, and adverse events in patients undergoing esophageal stent placement for benign indications: a large multicenter study. J Clin Gastroenterol. 2016; 50:373–378. PMID:
26905604.
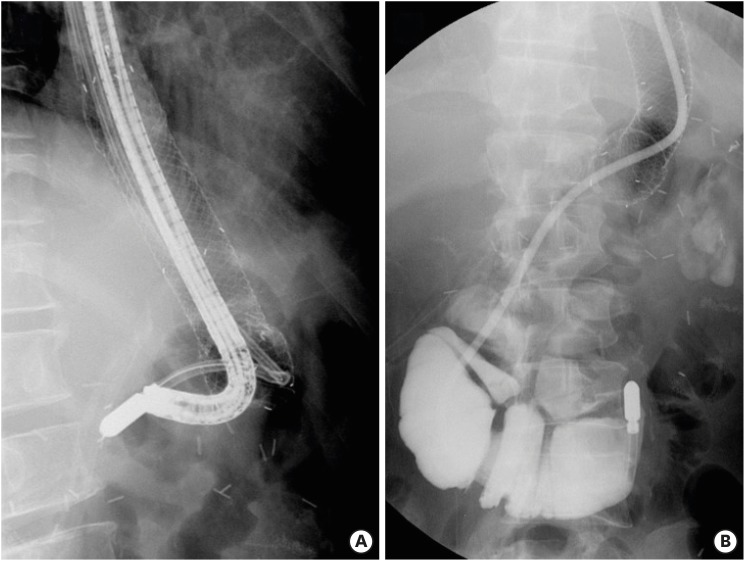
10. Shim CN, Kim HI, Hyung WJ, Noh SH, Song MK, Kang DR, et al. Self-expanding metal stents or nonstent endoscopic therapy: which is better for anastomotic leaks after total gastrectomy? Surg Endosc. 2014; 28:833–840. PMID:
24114516.

11. Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, et al. Full covered self-expandable metal stents for the treatment of anastomotic leak using a silk thread. Medicine (Baltimore). 2017; 96:e7439. PMID:
28723752.

12. van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011; 33:1292–1301. PMID:
21517921.

13. Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009; 23:1526–1530. PMID:
19301070.

14. van Halsema EE, Wong Kee Song LM, Baron TH, Siersema PD, Vleggaar FP, Ginsberg GG, et al. Safety of endoscopic removal of self-expandable stents after treatment of benign esophageal diseases. Gastrointest Endosc. 2013; 77:18–28. PMID:
23261092.

15. Wadhwa RP, Kozarek RA, France RE, Brandabur JJ, Gluck M, Low DE, et al. Use of self-expandable metallic stents in benign GI diseases. Gastrointest Endosc. 2003; 58:207–212. PMID:
12872087.

16. Chaput U, Heresbach D, Audureau E, Vanbiervliet G, Gaudric M, Bichard P, et al. Comparison of a standard fully covered stent with a super-thick silicone-covered stent for the treatment of refractory esophageal benign strictures: a prospective multicenter study. United European Gastroenterol J. 2013; 1:93–102.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download