1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
2. Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002; 5:177–182.
3. Pinson P, Joos G, Watripont P, Brusselle G, Pauwels R. Serum neuron-specific enolase as a tumor marker in the diagnosis and follow-up of small-cell lung cancer. Respiration. 1997; 64:102–107.
4. Cooper EH. Neuron-specific enolase. Int J Biol Markers. 1994; 9:205–210.
5. Kaiser E, Kuzmits R, Pregant P, Burghuber O, Worofka W. Clinical biochemistry of neuron specific enolase. Clin Chim Acta. 1989; 183:13–31.
6. Jørgensen LG, Osterlind K, Hansen HH, Cooper EH. Serum neuron-specific enolase (S-NSE) in progressive small-cell lung cancer (SCLC). Br J Cancer. 1994; 70:759–761.
7. Kimura M, Tsuda H, Morita D, Ichikura T, Ogata S, Aida S, et al. A proposal for diagnostically meaningful criteria to classify increased epidermal growth factor receptor and c-erbB-2 gene copy numbers in gastric carcinoma, based on correlation of fluorescence in situ hybridization and immunohistochemical measurements. Virchows Arch. 2004; 445:255–262.
8. Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ, et al. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007; 7:155–167.
9. Cho HJ, Baek KE, Park SM, Kim IK, Choi YL, Cho HJ, et al. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin Cancer Res. 2009; 15:2612–2619.
10. Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008; 52:738–746.
11. Cho JY. Molecular diagnosis for personalized target therapy in gastric cancer. J Gastric Cancer. 2013; 13:129–135.
12. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697.
13. Corso G, Pedrazzani C, Marrelli D, Pascale V, Pinto E, Roviello F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg. 2009; 144:722–727.
14. Park YS, Jin MY, Kim YJ, Yook JH, Kim BS, Jang SJ. The global histone modification pattern correlates with cancer recurrence and overall survival in gastric adenocarcinoma. Ann Surg Oncol. 2008; 15:1968–1976.
15. Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, et al. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010; 295:144–153.
16. Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, et al. p21 expression is a prognostic factor in patients with p53-negative gastric cancer. Cancer Lett. 2000; 148:181–188.
17. Yu D, Du K, Liu T, Chen G. Prognostic value of tumor markers, NSE, CA125 and SCC, in operable NSCLC Patients. Int J Mol Sci. 2013; 14:11145–11156.
18. Dong Y, Zheng X, Yang Z, Sun M, Zhang G, An X, et al. Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of nonsmall cell lung cancer. J Cancer Res Ther. 2016; 12:34–36.
19. Sosonkina N, Hong SK, Starenki D, Park JI. Kinome sequencing reveals RET G691S polymorphism in human neuroendocrine lung cancer cell lines. Genes Genomics. 2014; 36:829–841.
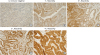
20. Lee HS, Kim WH. Tissue array methods for high-throughput clinicopathologic research. Cancer Res Treat. 2006; 38:1–6.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download