Abstract
Purpose
PIK3CA is often mutated in a variety of malignancies, including colon, gastric, ovary, breast, and brain tumors. We investigated PIK3CA expression in gastric cancer and explored the relationships between the PIK3CA expression level and clinicopathological features as well as survival of the patients.
Materials and Methods
We examined PIK3CA expression in a tissue microarray of 178 gastric adenocarcinomas by immunohisto-chemistry and reviewed patients' medical records.
Results
In our study, 112 of the 178 gastric cancer patients displayed positive PIK3CA expression. Overexpression of PIK3CA was correlated with low grade differentiation (P=0.001), frequent lymphatic invasion (P=0.032), and high T stage (P=0.040). Patients with positive PIK3CA staining were more likely to display worse overall survival rate than those with negative PIK3CA staining, as determined by Kaplan-Meier survival analysis with log-rank test (P=0.047) and a univariate analysis using the Cox proportional hazard model (hazard ratio=1.832, P=0.051).
Gastric cancer is the fifth most common malignant tumor and the third leading cause of cancer-related death worldwide. Although incidence of gastric cancer has decreased in recent years, it remains a major global health problem, with a particularly high prevalence rate in Asia.12 Improvements in surgical and chemotherapeutic interventions have led to lower gastric cancer morbidity and mortality rates; however, approximately half of gastric cancer patients are not cured.2 Both environmental factors and genetic/epigenetic alterations are thought to be associated with tumor development and progression, but the precise pathogenesis has not been determined.3 Therefore, the discovery of gastric cancer-specific markers that are clinically applicable for early cancer detection and targeted therapeutics is crucial.
The phosphatidylinositol 3-kinase (PI3K) signaling pathway is pivotal to drive the extracellular growth signaling during tumorigenesis, progression, and drug resistance of breast, colorectal, endometrial, and gastric cancers.34567 During malignant transformation, various genetic alterations may occur in any of the PI3K pathway components, such as the receptor tyrosine kinase genes, EGFR, HER2, KIT, PTEN, PIK3CA, and AKT. PI3K is a heterodimer comprising of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit that possess an enzymatic activity. To date, four isoforms of PI3K catalytic subunit (α, β, γ, and δ) have been identified. Among these, the gene encoding the α-isoform catalytic subunit (PIK3CA, p110α) was found to be frequently mutated or amplified, leading to the acquisition of oncogenic properties or increased tumor aggressiveness.89 PIK3CA mutation and amplification in gastric cancer have also been described; in particular, the amplification of PIK3CA is frequently obserevd in gastric adenocarcinoma.1011
PIK3CA mutation and amplification in gastric cancer can act as a driver mutation, and they are associated with carcinogenesis and adverse prognosis.1213 In gastric cancer, dysregulation of the PI3K-Akt pathway due to PIK3CA mutation and inactivation of the phosphatase and tensin homolog (PTEN) has been identified as a mechanism for trastuzumab resistance.14 Moreover, aberrant increase in PIK3CA immunoreactivity can promote gastric cancer metastasis.9 Here, we investigated PIK3CA expression in human gastric cancer tissue using immunohistochemistry. We evaluated the relationships between PIK3CA expression level and the conventional clinicopathological parameters, as well as effects on patient survival. In addition, we sought to determine the use of PIK3CA immunohistochemistry to predict the clinical outcome of gastric cancer.
For this retrospective study, we enrolled a consecutive series of 178 gastric cancer patients. Gastric adenocarcinoma was confirmed using histology for all patients. All patients underwent total or subtotal gastrectomy at the Soonchunhyang University Cheonan Hospital between July 2006 and December 2012. The patients had a mean age of 61 years (range, 27 to 81 years). They were regularly followed every 6 months or yearly after surgery, and their mean follow-up period was 58 months (range, 0 to 95 months). Among 178 patients, 66 patients (37.1%) died and 112 patients (66.9%) remain alive.
All tissue samples obtained in the study were fixed in formalin and embedded in paraffin. Medical records from the initial and follow-up visits, including clinical data, pathology reports, and hematoxylin and eosin (H&E)-stained slides were reviewed to confirm diagnoses. The clinicopathological variables of the patients, including age, sex, tumor size, tumor location, invasion depth, lymph node metastasis, distant metastasis, TNM stage, differentiation, lymphatic, vascular, and perineural invasions, Lauren and Ming classifications, and patient survival were also reviewed. Patients who received neoadjuvant chemotherapy and/or had other critical medical conditions were excluded in this study. Cases with no tissue samples available were also excluded.
Tissue microarrays (TMA) were prepared as previously described. 15 H&E-stained slides were evaluated under light microscopy to define representative and non-necrotic areas in the tumors. Single tissue cores (2 mm in diameter) were obtained from paraffin blocks (donor block) and transferred to a recipient block using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Cores of non-tumorous gastric mucosa tissue were included and assembled between the tumor cores.
For PIK3CA immunohistochemistry, 4-µm sections were cut from the TMA block and transferred onto coated slides. The sections were deparaffinized through incubation in a dry oven at 60℃ for 1 hour and three washes in xylene. Following block-age of the endogenous peroxidase activity using 5% hydrogen peroxide in methanol for 15 minutes at 37℃, the sections were autoclaved in a pH-6.0 epitope retrieval solution for 20 minutes for antigen retrieval. The sections were stained with a 1:75 dilution of rabbit polyclonal anti-PIK3CA antibody (HPA009985; Sigma-Aldrich, St. Louis, MO, USA). Following incubation in a humidified chamber at 4℃ for 16 hours, a secondary antibody treated using the BOND Polymer Refine Detection kit (Leica Biosystems, Wetzlar, Germany) was applied. Diaminobenzidine was used as chromogen for color development.
PIK3CA immunostaining was evaluated by two independent pathologists who were blinded to the clinicopathological information of the patients. Discrepancy in findings was solved by reviewing the cases using a multi-head microscope until consensus was reached. Cytoplasmic granular staining of PIK3CA was considered as immunoreactivity. Adjacent non-tumorous mucosa in the tumor core and normal gastric mucosa cores were used as negative controls. PIK3CA expression was graded based on the proportion and intensity of staining. The stained cell proportion was scored as: 0 (<5%); 1 (5% to 25%); 2 (26% to 50%); and 3 (>50%). Intensity of the immunostaining was scored as follows: 0 (negative); 1+ (weak positive); 2+ (moderate positive); and 3+ (strong positive) (Fig. 1). The final score was calculated by multiplying the proportion score with the intensity score. Final scores of <4 were considered negative, whereas scores of ≥4 were considered positive.16 For negative controls, 18 normal gastric mucosa cores were included in the TMA blocks. PIK3CA immunoreactivity score of these cores ranged from 0 to 3. Additionally, non-tumorous mucosa adjacent to the tumor core showed no immunoreactivity or weak PIK3CA-positive staining in contrast with the tumor glands(Fig. 2).
All statistical analyses were performed using the PASW ver. 18.0 statistical software (IBM Co., Armonk, NY, USA). The χ2 test for linear trends and Fisher's exact test were utilized to determine the relationships between PIK3CA expression and clinicopathological features of the patients. Survival curves were generated using the Kaplan-Meier method with log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards regression method to compare differences in overall survival (OS) between patient subgroups. P<05.0 was considered statistically significant.
In the current study, positive PIK3CA staining was observed in 112 gastric cancer patients (62.9%). PIK3CA overexpression was significantly correlated with frequent low-grade differentiation (well/moderately differentiated; P=0.001), intestinal-type histology of the Lauren classification (P=0.012), lymphatic invasion (P=0.032), and advanced T stage (pT3 or more; P=0.040). Elevated PIK3CA expression was also more likely to be observed in the upper third of the stomach (P=0.182) and display later onset age (P=0.051); however, these correlations were not statistically significant. No significant associations were found for other clinicopathological parameters, including sex (P=0.289),the Ming classification (P=0.512), lymph node metastasis (P=0.202), and the presence of vascular or perineural invasion (P=0.863). Table 1 summarizes the correlations between PIK3CA expression and the clinicopathological parameters of the patients.
A significant correlation between PIK3CA overexpression and poor survival was found using the Kaplan-Meier survival analysis with log-rank test. Patients that displayed positive PIK-3CA staining had worse OS than those with negative PIK3CA staining (P=0.047; Fig. 3). To further evaluate the prognostic implications of PIK3CA expression in gastric cancers, a univariate analysis using the Cox proportional hazard regression model was performed. In the univariate analysis, patients with positive PIK3CA staining were more likely to display reduced OS compared to those in the PIK3CA-negative group (P=0.051; hazard ratio=1.832; Table 2). No significant differences in OS were found between PIK3CA-positive and negative groups using a multivariate analysis (Table 3).
PI3K is a component of the PI3K signaling pathway that plays crucial roles in the regulation of cell proliferation, survival, and adhesion capacity, and is frequently upregulated in human cancers.1718 PIK3CA, encoding the p110α subunit of PI3K, is located on chromosome 3q26.3 and mutations of this gene are common in various malignant tumors, including colon, gastric, ovary, breast, and brain tumors.11181920 In gastric cancer, many studies have been performed to evaluate the role of somatic PIK3CA mutations, but these studies focused less on the aberrant overexpression of PIK3CA. However, significance of PIK3CA overexpression in other malignant tumors, including breast, esophageal and colorectal cancers, has been evaluated by immunohistochemistry.81621 Increased PIK3CA immunoreactivity has been implicated for poor prognosis and multi-drug resistance in other cancers, suggesting a promising use of PIK3CA expression, as assessed by immunohistochemistry, as a cancer biomarker.81621 Furthermore, a recent study that evaluated a PIK3CA mutation in esophageal cancer revealed that PIK3CA overexpression, determined by immunohistochemistry, could be utilized as an independent factor to predict high local recurrence.21 To the best of our knowledge, the current study is second to report PIK3CA expression using immunohistochemistry and first to describe the significance of PIK3CA overexpression for gastric cancer patient survival.
In an in vitro study using a tongue cancer cell line, inhibition of PIK3CA expression was shown to suppress PTEN and Akt phosphorylations, resulting in decreased proliferative activity and invasiveness and increased drug sensitivity.22 In gastric cancer cell lines, knockdown of PIK3CA by siRNA also led to reduced tumor cell invasion and migration, suggesting that PIK3CA expression is involved in cancer cell invasion.23 In this study, we found that PIK3CA upregulation was associated with tumor cell invasion. PIK3CA expression level was associated with tumor T stage, which represents the invasion depth of tumor cells in viscous hollow organs including the stomach. The invasion depth of tumor cells can be categorized as superficial invasion (T1 and T2) and deep invasion (T3 and T4). We observed a statistically significant difference in PIK3CA expression between these categories (P=0.040). Furthermore, an increase in lymphatic vessel invasion frequency was observed in the PIK3CA-positive group (P=0.032). Because lymphatic vessel invasion can indicate tumor cell invasiveness, this observation also supports the hypothesis that PIK3CA expression induces tumor invasiveness in gastric cancer. Therefore, our results obtained using human cancer tissues are in agreement with previous in vitro findings.
PIK3CA can also serve as a determinant for tumor differentiation or morphological phenotypes. In a breast cancer study, increased PIK3CA immunoreactivity was associated with high-grade morphology, as well as different histology subtypes (invasive ductal carcinoma versus other histology types).8 A previous colorectal cancer study also reported PIK3CA overexpression in specimens with high grade histology and mucinous adenocarcinoma morphology.1624 In contrast to previous studies in other organs, we observed PIK3CA overexpression in carcinomas with low grade histology (well/moderately differentiated; P=0.040). This trend was also observed when association between the Lauren classification and PIK3CA expression was determined. We observed positive PIK3CA staining in intestinal-type adenocarcinomas, and negative staining in mixed- and diffuse-type carcinomas (P=0.001). The Lauren classification subdivides gastric cancers into diffuse- and intestinal-types based on their morphology.25 In contrast, Tan et al.26 reported a molecular classification of gastric cancers based on the gene expression patterns. In their study, mesenchymal- and proliferative-types were associated with diffuse- and intestinal-type adenocarcinomas, respectively. Furthermore, a recent study revealed that each Lauren classification subtype (diffuse, mixed, and intestinal) exhibited a unique DNA methylation signature during early gastric carcinogenesis.27 These findings suggested that the morphological characteristics of gastric cancer are induced by a variety of unique genetic or epigenetic alterations. Therefore, we concluded that different PIK3CA expression levels among the histological subgroups can partially explain their morphological features, including tumor differentiation. However, the precise mechanism is yet to be defined.
Few studies have investigated the correlation between PIK3CA immunoreactivity and patient survival in gastric cancer. Several breast and colorectal cancer studies demonstrated that elevated PIK3CA expression, as observed using immunohistochemistry, was correlated with worse OS.821 Another study using a small cohort of 53 primary gastric cancer cases revealed that PIK3CA overexpression was a predictive marker for metastasis in gastric cancers; however, survival analysis was not performed.9 We found that the PIK3CA-positive group was more likely to show worse OS than the negative group using a log-rank test and univariate survival analysis (P=0.047 and P=0.051, respectively). These results suggested that the level of PIK3CA expression can reflect the prognosis of gastric cancer patients.
To summarize, PIK3CA overexpression was associated with poor survival and aggressive clinicopathological features, such as deep invasion of the gastric wall (high T stage) and lymphatic invasion. PIK3CA expression, assessed by immunohistochemistry, was observed in intestinal-type adenocarcinoma, suggesting PIK3CA role as a determinant for molecular and morphological characteristics of gastric cancer. In conclusion, PIK3CA immunochemistry may serve as a useful method to predict patient survival and aggressiveness of gastric cancer.
Figures and Tables
Fig. 1
Representative PIK3CA immunohistochemical staining images of gastric cancers (×400) exhibiting intensity score of 0 (A), 1 (B), 2 (C), or 3 (D).
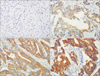
Fig. 2
(A, B) Normal gastric mucosa, which was used as a negative control, displayed absence of PIK3CA staining (A) or weak PIK3CA staining (B) (×200). (C) Positive PIK3CA staining in tumor glands and negative staining in the adjacent non-tumorous gastric mucosa (×200).

Fig. 3
Survival curve of the low PIK3CA expression group (n=112) and high PIK3CA group (n=66) as assessed by Kaplan-Meier survival analysis with log-rank test.

Table 1
Correlations between PIK3CA expression and various clinicopathological features in gastric cancer patients

References
1. Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, et al. Clinicopathological characteristics and survival outcomes of primary signet ring cell carcinoma in the stomach: retrospective analysis of single center database. PLoS One. 2015; 10:e0144420.
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
3. Ibarrola-Villava M, Llorca-Cardeñosa MJ, Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, et al. Deregulation of ARID1A, CDH1, cMET and PIK3CA and target-related microRNA expression in gastric cancer. Oncotarget. 2015; 6:26935–26945.
4. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296:1655–1657.
5. Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005; 65:10669–10673.
6. Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008; 68:1953–1961.
7. Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008; 27:5497–5510.
8. Aleskandarany MA, Rakha EA, Ahmed MA, Powe DG, Paish EC, Macmillan RD, et al. PIK3CA expression in invasive breast cancer: a biomarker of poor prognosis. Breast Cancer Res Treat. 2010; 122:45–53.
9. Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, et al. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol. 2010; 16:4986–4991.
10. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006; 7:606–619.
11. Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005; 5:29.
12. Shi J, Yao D, Liu W, Wang N, Lv H, Zhang G, et al. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC Cancer. 2012; 12:50.
13. Fassan M, Simbolo M, Bria E, Mafficini A, Pilotto S, Capelli P, et al. High-throughput mutation profiling identifies novel molecular dysregulation in high-grade intraepithelial neoplasia and early gastric cancers. Gastric Cancer. 2014; 17:442–449.
14. Sukawa Y, Yamamoto H, Nosho K, Ito M, Igarashi H, Naito T, et al. HER2 expression and PI3K-Akt pathway alterations in gastric cancer. Digestion. 2014; 89:12–17.
15. Kim KJ, Lee KS, Cho HJ, Kim YH, Yang HK, Kim WH, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014; 45:285–293.
16. Wen F, He S, Sun C, Li T, Wu S. PIK3CA and PIK3CB expression and relationship with multidrug resistance in colorectal carcinoma. Int J Clin Exp Pathol. 2014; 7:8295–8303.
17. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501.
18. Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004; 304:554.
19. Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005; 11:2875–2878.
20. Lin Y, Jiang X, Shen Y, Li M, Ma H, Xing M, et al. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009; 16:301–310.
21. Wang L, Shan L, Zhang S, Ying J, Xue L, Yuan Y, et al. PIK3CA gene mutations and overexpression: implications for prognostic biomarker and therapeutic target in Chinese esophageal squamous cell carcinoma. PLoS One. 2014; 9:e103021.
22. Chen Y, Hou Q, Yan W, Luo J, Chen D, Liu Z, et al. PIK3CA is critical for the proliferation, invasiveness, and drug resistance of human tongue carcinoma cells. Oncol Res. 2011; 19:563–571.
23. Zhou XK, Tang SS, Yi G, Hou M, Chen JH, Yang B, et al. RNAi knockdown of PIK3CA preferentially inhibits invasion of mutant PIK3CA cells. World J Gastroenterol. 2011; 17:3700–3708.
24. Hechtman JF, Sadowska J, Huse JT, Borsu L, Yaeger R, Shia J, et al. AKT1 E17K in colorectal carcinoma os associated with BRAF V600E but not MSI-H status: a clinicopathologic comparison to PIK3CA helical and kinase domain mutants. Mol Cancer Res. 2015; 13:1003–1008.
25. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49.
26. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011; 141:476–485.
27. Chong Y, Mia-Jan K, Ryu H, Abdul-Ghafar J, Munkhdelger J, Lkhagvadorj S, et al. DNA methylation status of a distinctively different subset of genes is associated with each histologic Lauren classification subtype in early gastric carcinogenesis. Oncol Rep. 2014; 31:2535–2544.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download