Abstract
Gastrointestinal stromal tumors (GISTs) are the most common primary mesenchymal neoplasms of the gastrointestinal tract and usually appear as a well-circumscribed mass. However, it may be difficult to confirm the extent of the disease for some GISTs. A 70-year-old asymptomatic female presented for a regular physical exam. An esophagogastroduodenoscopy showed a 2.0 cm protruding mass on the gastric fundus. Endoscopic ultrasound revealed an ill-defined heterogenous hypoechoic lesion (3.0×1.5 cm). A computed tomography (CT) scan demonstrated a 4.5 cm multifocal calcified mass at the gastric body as well as at the gastric fundus. Laparoscopic gastric wedge resection was performed according to the extent of multifocal calcifications that are shown on the CT. Intraoperative specimen mammography and intraoperative biopsy might be helpful to obtain a tumor-free margin. Final pathologic diagnosis was an intermediate risk GIST in multilobular form. In patients with diffuse multifocal calcifications in the stomach, the possibility of GIST should be considered.
Gastrointestinal stromal tumors (GISTs) are the most common primary mesenchymal neoplasms of the gastrointestinal tract.1 Although GISTs may occur throughout the gastrointestinal tract, approximately 60% of them originate in the stomach and 30% in the small intestine.2 GISTs usually appear as a discrete well-circumscribed but non-encapsulated mass with variable gross appearances, growing as intraluminal, extraluminal, or combination forms.3 Some GISTs present with circumscribed, mottled, or patchy calcifications similar to other tumors such as gastric cancer and hemangiomas with phleboliths.4 However, to our knowledge, GISTs showing a multilobular form with diffuse multifocal calcifications have not yet been reported. Here we report a case of gastric GIST presenting with a multilobular growth pattern with diffuse multifocal calcifications.
A 70-year-old female without a significant medical history visited Eulji University Hospital for a regular physical exam. She had denied any symptoms such as abdominal pain, melena, anorexia, or weight loss. The physical examination was unremarkable. Laboratory examination showed no abnormalities. An esophagogastroduodenoscopy showed a 2.0×2.0 cm protruding mass with normal overlying mucosa at the fundus in the stomach (Fig. 1A). Endoscopic ultrasound (EUS) revealed an ill-defined heterogenous hypoechoic lesion (3.0×1.5 cm) with multiple hyperechoic spots, arising from the muscularis propria layer (Fig. 1B). A computed tomography (CT) scan of the abdomen demonstrated a 4.5 cm multifocal calcified mass at the gastric body as well as at the gastric fundus (Fig. 2A, B). There were no enlarged perigastric or periesophageal lymph nodes. A preoperative evaluation led to the possible diagnosis of multifocal hemangiomas with phleboliths or a calcified GIST. A laparoscopic gastric wedge resection of the gastric fundus and high body was performed using linear stapler according to the maximal extent of multifocal calcifications that was shown on CT for tumor-free margin due to indistinct boundary of the tumor and discordant finding between endoscopy and CT (Fig. 2C). In addition, intraoperative mammography and frozen biopsy of the specimen was performed to confirm the complete removal of the excised specimen including the multifocal calcifications, and the specimen was compared to the preoperative CT finding (Fig. 2D). Calcification on the intraoperative specimen mammography was measured at 5.4×1.9 cm. The resected specimen revealed a well-circumscribed elongated mass measuring 5.2×2.0 cm, showing solid, whitish-yellow parenchyma (Fig. 3A). Histologically, the tumor that originated from the muscularis propria was extended longitudinally in multilobular form with diffuse calcifications (Fig. 3B). The tumor was composed of admixed spindle and epithelioid cells displaying finely vesicular chromatin and palely staining cytoplasm arranged in short intersecting fascicles and diffuse sheets (Fig. 3C). The mitotic rate was less than 5 mitoses per 50 high power fields and no prominent sign of nuclear atypia was seen. There was mild pleomorphism without necrosis. On immunohistochemical staining, the tumor cells were strongly positive for CD117 (Fig. 3D) and CD34, and negative for S-100 protein, alpha-smooth muscle actin, and desmin, and weakly positive for the DOG1 antibody. Since the size of this tumor was 5.2 cm (5~10 cm), the GIST was deemed an intermediate risk according to risk stratification guidelines.5 The postoperative recovery was uneventful and the patient was discharged eight days later.
GISTs are currently thought to originate from the gastrointestinal pacemaker cells, the interstitial cells of Cajal, which are embedded in the musculature of the gastrointestinal tract. The majority of GISTs generally appear to be well-defined ovoid or round shaped masses, regardless of the size of the tumor. However, in our case, the GIST shows an ill-defined mass presented with a longitudinal and diffuse growth pattern along the muscular layer. Therefore, we can only presume the extent of the tumor through the calcification distribution due to the indistinct boundary of the tumor.
Endoscopic examination of a GIST generally shows a smooth subepithelial mass displacing the overlying mucosa and is useful to distinguish it from common gastrointestinal tract tumors, which usually originate in the mucosa. EUS is useful for estimation of size, origin, and invasion depth of the GIST. CT is considered to be the imaging modality of choice for the detection, staging, surgical planning, and follow-up of patients with GIST.6 However, in our case, the extent of the tumor could not be precisely localized through endoscopy, EUS, and CT due to the unusual longitudinal tumor growth pattern, with replacement of the muscularis propria. The only evidence for presuming the range of the tumor was the distribution of calcification shown on CT imaging.
Calcification is not rare in GISTs and is found in 3% of cases.4 It may also be present in other gastrointestinal tumors like mucinous gastric cancer and hemangiomas with phleboliths.7 Most cases of primary GIST with calcification that have been reported showed a solitary, punctuate, mottled, or patchy calcification pattern. 8 However, extensive dense calcification throughout the tumor 4 or diffuse multifocal distribution of calcification of GISTs is very rare in our case. Rege et al.9 reported that multifocal tumors with multinodular growth patterns typically arise in pediatric GISTs, not adult cases. No known relation currently exists between multifocal calcification and multilobular growth patterns.
The principles of surgical treatment for primary resectable GISTs are complete resection without causing tumor rupture, while acquiring negative margins.10 It is extremely important to avoid tumor rupture because it is associated with very poor outcomes, even in small GISTs with low mitotic counts.11 Generally, it is not difficult to decide the extent of resection because most cases show a well-circumscribed margin. However, in our case, we used the extent of the calcifications shown on CT as the only indicator for the smaller extent of resection, due to the unusual longitudinal growth pattern of GISTs.
In conclusion, we herein present the rare case of a gastric GIST showing a mutilobular form with diffuse multifocal calcifications, which was successfully treated by laparoscopic gastric wedge resection. In the case of an indistinct tumor boundary with calcification, intraoperative specimen mammography and frozen biopsy might be helpful to obtain a tumor-free resection margin. Additionally, in patients with diffuse multifocal calcifications of the stomach, the possibility of a GIST should be considered for the differential diagnosis.
Figures and Tables
Fig. 1
Endoscopic findings of the stomach. (A) Esophagogastroduodenoscopy image demonstrates a 2 cm subepithelial protruding mass located in the fundus of the stomach, covered by normal mucosa. (B) Endoscopic ultrasound revealed an ill-defined heterogenous hypoechoic lesion (3.0×1.5 cm) with multiple hyperechoic spots, arising from the muscularis propria layer.
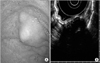
Fig. 2
Radiologic findings of the stomach. (A, B) Contrast-enhanced axial and sagittal computed tomography examination demonstrating thick or patchy multifocal calcifications. (C) The schematic drawing of the gastric wedge resection showing the resected stomach (dotted line), calcifications (variable sized dots), stomach (S), spleen (SP), and short gastric vessels (SG). (D) Intraoperative specimen mammography showing diffuse multifocal variable sized calcifications of the gastric fundus and high body along the greater curvature.
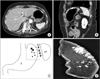
Fig. 3
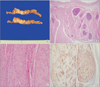
Pathologic findings. (A) Gross resection of the tumor revealed a solid, whitish-yellow parenchyma with multifocal diffuse calcifications (cut section of gastrointestinal stromal tumor). (B) Microscopically, the tumor originated from the muscularis propria and extended longitudinally in a multilobular form with diffuse calcifications (H&E, ×20). (C) The sliced surface of the tumor was characterized by spindled and epithelioid mixed tumor cells (H&E, ×40). (D) Immunohistochemically, the tumor cells were positive for CD117 (c-kit [CD117] immunohistochemical stain, ×200).
References
1. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000; 15:1293–1301.
2. Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010; 25:853–862.
3. Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically reevaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006; 391:322–329.
4. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23:283–304.
5. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.
6. Chourmouzi D, Sinakos E, Papalavrentios L, Akriviadis E, Drevelegas A. Gastrointestinal stromal tumors: a pictorial review. J Gastrointestin Liver Dis. 2009; 18:379–383.
7. Ghahremani GG, Meyers MA, Port RB. Calcified primary tumors of the gastrointestinal tract. Gastrointest Radiol. 1978; 2:331–339.
8. Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003; 13:1669–1678.
9. Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. "Pediatric-type" gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011; 35:495–504.
10. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.
11. Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Woźniak A, Limon J, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007; 14:2018–2127.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download