Abstract
Pregnancy-associated gastric cancer is extremely rare. In many cases, it is diagnosed at an advanced stage because the symptoms during pregnancy are generally overlooked. We report three cases of gastric cancer during pregnancy with various outcomes. The first case included a patient with stage IV gastric cancer who received palliative chemotherapy. This patient had a preterm birth and died 7 months after diagnosis. The second case received neoadjuvant chemotherapy during pregnancy and a total gastrectomy was performed after delivery. She then received adjuvant chemoradiotherapy. This patient developed pulmonary metastasis and died of recurrence 41 months after surgery. In the third case, a distal subtotal gastrectomy was performed at week 14 of pregnancy, with no complications. The patient received adjuvant chemoradiotherapy. She is currently without recurrence 14 months after surgery. In patients with pregnancy-associated gastric cancer, treatment decisions are predominantly influenced by clinical stage and gestational age at diagnosis.
Pregnancy-associated gastric cancer is rare, occurring in only 0.025% to 0.1% of all pregnancies.1 Gastric cancer diagnosed during pregnancy can be a devastating situation for the mother and the fetus, and patients with gastric cancer diagnosed during pregnancy have a dismal prognosis.2 In many cases pregnancy-associated gastric cancer is diagnosed at an advanced stage and only 45% to 56% of patients undergo surgical resection.3 The diagnosis of this type of cancer is difficult because symptoms such as nausea, vomiting, or abdominal discomfort are generally overlooked during pregnancy.4 We report three cases of gastric cancer during pregnancy with different outcomes.
In Table 1, we present a summary of the clinical characteristics, treatment, and outcomes of each patient.
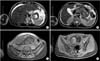
A 27-year-old woman, gravida 3, para 2, was admitted with a 1-month history of epigastric pain and weight loss (7 kg). At the time of admission, she was 12 weeks pregnant. Esophagogastroduodenoscopy (EGD) was performed, revealing a large Borrmann type IV gastric tumor. A biopsy was performed, and a diagnosis of poorly differentiated adenocarcinoma with signet-ring cell morphology was made. Magnetic resonance imaging revealed thickening of the gastric wall, metastatic celiac axis lymph nodes, and peritoneal dissemination (Fig. 1). The patient's case was discussed during a tumor board meeting with surgical, medical oncology, and obstetrics specialists. It was determined that the patient should be treated with palliative chemotherapy with 5-fluorouracil (5-FU) and cisplatin. The patient underwent four chemotherapy cycles of 5-FU (1,000 mg/m2, 24 hours infusion) on days 1, 2, 3, 4 and cisplatin (75 mg/m2) on day 1. This regimen was repeated every 28 days. Fourteen weeks after diagnosis, at week 26 of the pregnancy, the patient had a spontaneous delivery. Her son weighed 850 grams, and had an Apgar score of 3/5. The newborn required ventilatory support due to respiratory failure, renal and cardiac insufficiency, and he died 15 days after birth. The mother died 7 months after her diagnosis.
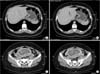
A 33-year-old woman, gravida 2, para 1, was admitted to the emergency department with a 2-month history of abdominal pain and weight loss (21 kg). EGD was performed, revealing an extensive ulcer in the stomach. Biopsy results revealed a poorly differentiated adenocarcinoma with signet-ring cell morphology. An abdominopelvic computed tomography (CT) scan revealed gastric wall thickening and a gravid uterus, with no signs of dissemination (Fig. 2). Ultrasonography (US) confirmed a 15-week pregnancy. We recommended immediate surgery, but the patient refused due to concerns regarding the risk of perioperative abortion. The medical team, in agreement with the patient, decided on neoadjuvant chemotherapy with four cycles of FOLFOX4. Chemotherapy was started in week 18 of the pregnancy. The patient was treated with FOLFOX4 as follows: intravenous (IV) administration of 85 mg/m2 oxaliplatin, 200 mg/m2 leucovorin, and IV push administration of 400 mg/m2 5-FU on day 1, and 600 mg/m2 5-FU IV continuous infusion for 24 hours on days 1 and 2. This regimen was repeated every 14 days.
At week 36 of the pregnancy, the patient went into spontaneous delivery, and gave birth to a male infant weighing 3.150 kg, with an Apgar score of 9/9. The healthy newborn was discharged 3 days after birth. One month after delivery, the patient underwent a radical total gastrectomy with D2 lymph node dissection. Histopathological examination of the resected specimen revealed serosal involvement of the gastric wall and lymph node metastasis in 15 out of 31 resected nodes. The pathologic stage was pIIIC (T4aN3M0, Union for International Cancer Control/American Joint Committee on Cancer [UICC/AJCC] 7th edition). The patient was discharged 7 days after surgery without postoperative complications. She received adjuvant chemoradiotherapy as described in the study by Macdonald and colleagues.5 The regimen consisted of 5-FU (425 mg/m2/day) and leucovorin (20 mg/m2/day) for 5 days, followed by radiation to a total of 45 Gy (1.8 Gy per day, 5 days/week for 5 weeks) beginning on day 28, with 5-FU (400 mg/m2/day) and leucovorin (20 mg/m2/day) for the first 4 days and the last 3 days of radiotherapy. One month after radiotherapy, two additional cycles of 5-FU and leucovorin were administered once every 28 days. However, the patient developed pulmonary metastasis and died of recurrence 41 months after surgery.
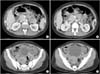
A 29-year-old woman, gravida 3, para 2, had a 1-year history of epigastric pain. A prepyloric ulcer was diagnosed on EGD. Biopsy revealed adenocarcinoma with signet-ring cell morphology. Abdominopelvic CT demonstrated prepyloric gastric wall thickening, with no signs of dissemination (Fig. 3). The patient had 6 weeks of amenorrhea. The results of a human chorionic gonadotropin blood test and an US confirmed that the patient was 6 weeks pregnant. Following the tumor board meeting, surgery during the second trimester was recommended. The medical team decided to perform surgery at week 14 of the pregnancy, to which the patient agreed. An open radical subtotal gastrectomy was performed with D2 lymph node dissection; R0 resection was achieved. Histopathological examination revealed subserosal involvement of the gastric wall and lymph node metastasis in 9 out of 25 resected nodes. The disease was classified as stage pIIIB (T3N3M0, AJCC/UICC 7th edition). The patient was discharged 9 days after surgery with no complications. After confirming adequate fetal development, an elective cesarean section was decided upon at week 32 of gestation, mainly to avoid further delays in the start of adjuvant treatment. A male infant weighing 1.750 kg, with an Apgar score of 8/9, was delivered. The patient received adjuvant chemoradiotherapy,5 as described in case 2 above. She is currently without recurrence 14 months after surgery.
Gastric carcinoma is rare in patients younger than 40 years old,6 and even rarer during pregnancy. Gastric carcinoma in young patients tends to be poorly differentiated, with an overall poor prognosis.3
The Helicobacter pylori infection rate is significantly higher in pregnant women than in non-pregnant women (26.6% versus 11%).7 Furthermore, the secretion of gastric acid decreases during pregnancy, while the production of gastric mucus increases. Histaminase produced by the placenta deactivates histamine function; therefore, the patient exhibits no deterioration of symptoms caused by a cancerous ulcer.8
The pathogenesis of pregnancy-related gastric cancer is a matter of discussion. Furukawa et al.9 suggested that pregnancy and/or delivery in young women accelerates the growth of stomach cancer, whereas Jaspers et al.10 suggested that clinical features and prognosis in gastric cancer during pregnancy did not differ from those in other young patients.
A Japanese review of 61 patients with gastric cancer diagnosed during pregnancy reported that only 47.5% of patients underwent surgery. The patients who underwent gastrectomy had a high incidence of in-hospital death (22.7%) and a poor prognosis, with a 3-year survival rate of 21.1%.11 The prognosis of gastric cancer during pregnancy is poor because most cases present at an advanced stage; the diagnosis is delayed because the symptoms are all nonspecific for cancer and are attributed to the pregnancy.12 In accordance with this, our three patients were diagnosed with stage III~IV disease.
Chemotherapy for unresectable gastric cancer during pregnancy is complicated because there are two aspects to consider. First is the importance of administering chemotherapy as soon as possible after diagnosis. Second is the importance of continuing the pregnancy as long as possible to ensure the safety of the fetus.13 Ueo et al.11 reported that treatment of pregnancy-associated gastric cancer depends on gestational age and the stage of the gastric cancer. Prior to 22 weeks of gestation, the mother should be treated after termination of the pregnancy by abortion. In Chile, abortion under any circumstances is prohibited by law; therefore, for this reason, this option could not be considered for our patients. In the first case, palliative chemotherapy was performed because the patient presented with stage IV gastric cancer. In the second and third cases, surgery was recommended in the second trimester. In the second case, neoadjuvant chemotherapy was administered after careful consideration owing to the mother's refusal to undergo surgery during pregnancy. Herein, it is difficult to draw firm conclusions, but both treatment strategies proved to be safe and allowed a R0 resection and a satisfactory fetal outcome.
The use of chemotherapy during the first trimester increases the risk of spontaneous abortion, fetal death, and major malformations; therefore, chemotherapeutic agents are not recommended during this period.14 In the second trimester, there are no major differences in the incidence of malformations between infants from normal pregnancies and those from pregnancies in which chemotherapy was administered.15 There is no standard chemotherapy regimen for treatment during pregnancy.
In conclusion, pregnancy-associated gastric cancer is extremely rare. Diagnosis is generally at an advanced stage due to symptoms that are frequently observed in a normal pregnancy. A multidisciplinary approach, with medical oncologists, surgeons, and obstetricians is essential for adequate therapeutic decision-making in this difficult and rare scenario. The treatment decisions should consider clinical stage and gestational age at diagnosis.
Figures and Tables
Fig. 1
Magnetic resonance imaging findings in case 1. (A, B) Gastric wall thickness, lymph node metastasis, perihepatic, and perisplenic fluid. (C, D) Gravid uterus.
References
1. Sakamoto K, Kanda T, Ohashi M, Kurabayashi T, Serikawa T, Matsunaga M, et al. Management of patients with pregnancy-associated gastric cancer in Japan: a mini-review. Int J Clin Oncol. 2009; 14:392–396.
2. Song MJ, Park YS, Song HJ, Park SJ, Ahn JY, Choi KD, et al. Prognosis of pregnancy-associated gastric cancer: an age-, sex-, and stage-matched case-control study. Gut Liver. 2016; 10:731–738.
3. Chong VH, Lim CC. Advanced disseminated gastric carcinoma in pregnancy. Singapore Med J. 2003; 44:471–472.
4. Chen Y, Li Y, Wang H, Lu J, Jin M, Zhang Z. Maternal gastric carcinoma with metastasis to the placenta: a case report. Oncol Lett. 2014; 8:2509–2510.
5. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730.
6. Rugge M, Busatto G, Cassaro M, Shiao YH, Russo V, Leandro G, et al. Patients younger than 40 years with gastric carcinoma: Helicobacter pylori genotype and associated gastritis phenotype. Cancer. 1999; 85:2506–2511.
7. Lanciers S, Despinasse B, Mehta DI, Blecker U. Increased susceptibility to Helicobacter pylori infection in pregnancy. Infect Dis Obstet Gynecol. 1999; 7:195–198.
8. Yoshida M, Matsuda H, Furuya K. Successful treatment of gastric cancer in pregnancy. Taiwan J Obstet Gynecol. 2009; 48:282–285.
9. Furukawa H, Iwanaga T, Hiratsuka M, Imaoka S, Ishikawa O, Kabuto T, et al. Gastric cancer in young adults: growth accelerating effect of pregnancy and delivery. J Surg Oncol. 1994; 55:3–6.
10. Jaspers VK, Gillessen A, Quakernack K. Gastric cancer in pregnancy: do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur J Obstet Gynecol Reprod Biol. 1999; 87:13–22.
11. Ueo H, Matsuoka H, Tamura S, Sato K, Tsunematsu Y, Kato T. Prognosis in gastric cancer associated with pregnancy. World J Surg. 1991; 15:293–297. discussion 298.
12. Cift T, Aydogan B, Akbaş M, Aydın B, Demirkiran F, Bakkaloglu DV, et al. Case report: gastric carcinoma diagnosed at the second trimester of pregnancy. Case Rep Obstet Gynecol. 2011; 2011:532854.
13. Nishie H, Mizushima T, Suzuki Y, Fukusada S, Inoue T, Kachi K, et al. Chemotherapy treatment of a pregnant woman with progressive gastric cancer. Intern Med. 2015; 54:1207–1212.
14. Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004; 5:283–291.
15. Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer. 2006; 42:126–140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download