Abstract
We report a unique case of synchronous double primary gastric cancer consisting of adenocarcinoma components with micropapillary features and composite glandular-endocrine cell carcinoma components. The patient was a 53-year-old man presenting with a 6-month history of epigastric pain and diarrhea. A subtotal gastrectomy was performed. Histologically, one tumor was composed of micropapillary carcinoma components (50%) with tight clusters of micropapillary aggregates lying in the empty spaces, admixed with moderately differentiated adenocarcinoma components. MUC-1 was expressed at the stromal edge of the micropapillary component. The other tumor was composed of atypical carcinoid-like neuroendocrine carcinoma (50%), adenocarcinoid (30%), and adenocarcinoma components (20%). The neuroendocrine components were positive for CD56, synaptophysin, chromogranin, and creatine kinase. The adenocarcinoid components were positive for both carcinoembryonic antigen and neuroendocrine markers (amphicrine differentiation). This case is unique, due to the peculiar histologic micropapillary pattern and the histologic spectrum of adenocarcinoma adenocarcinoid-neuroendocrine carcinoma of the synchronous composite tumor.
Gastric cancer is one of the most frequently reported malignant tumors in Korea.1 Cases of multiple synchronous or metachronous gastric cancers have been increasing in number during the last few decades, due to both the development of more sophisticated diagnostic tools and an increase in the number of elderly patients. The recently reported incidence of synchronous multiple gastric cancers is approximately 3.3% to 6.0%.23 We have experienced a unique case of adenocarcinoma with micropapillary features occurring synchronously with a composite glandular-endocrine cell carcinoma in the stomach.
A 53-year-old man presented with a 6-month history of epigastric pain and diarrhea to Gangneung Asan Hospital in May 2006. A chest radiograph and electrocardiography showed no abnormalities in the thoracic organs. Computed tomography of the abdomen revealed a mass-like lesion, measuring 3 cm in the anterior wall of the antrum. Colonoscopic findings were unremarkable. Endoscopy revealed no pathological finding in esophagus but showed two separate ulcerofungating and ulcerated lesions that had a tendency to bleed easily on touch in the anterior and posterior walls of the mid antrum. The remaining mucosa showed moderate atrophic change. The serum levels of carcinoembryonic antigen (CEA) and cancer antigen 19-9 were elevated to 8.1 ng/ml (normal range, 0~6 ng/ml) and 40.5 U/ml (normal range, 0~31 U/ml), respectively. Endoscopic biopsy revealed a histological diagnosis of poorly differentiated carcinoma. The patient underwent a subtotal gastrectomy with gastrojejunostomy (Billroth II).
On gross examination, the resected stomach showed two separate ulcerofungating (3.5×3.0×0.5 cm) and ulcerated tumors (3.0×2.8×0.8 cm) in the anterior and posterior walls of the mid antrum, respectively (Fig. 1). The tumors were located 3 cm apart from each other.
Histologically, the larger tumor was composed of an invasive micropapillary carcinoma component with tight clusters of micropapillary aggregates lying in the clear and empty spaces (50%) admixed with moderately differentiated adenocarcinoma components (50%; Fig. 2A, B). The tumor extended to the subserosa. Immunohistochemically, expression of epithelial membrane antigen (EMA) and MUC-1 (Fig. 2C) was observed at the stromal edge of the micropapillary tumor cells. These cells were also positive for CK7 and CEA expression, while they were negative for CK20, thyroid transcription factor 1, estrogen receptor, and progesterone receptor expression. The lining cells of most clear spaces around the micropapillary clusters of the tumor cells were negative for D2-40, CD34, and factor VIII-related antigen (Fig. 2D). A few true lympho-vascular tumor emboli were also noted in the peripheral portion of the tumor.
An intermixed area of the adenocarcinoma and goblet cell carcinoid was noted (Fig. 3A, B). The other tumor was composed of a solid sheet of atypical carcinoid-like, well-differentiated neuroendocrine carcinoma components (50%; Fig. 3C) showing frequent mitotic figures of more than 20/10 high-power fields and extensive necrosis, adenocarcinoid (goblet cell carcinoid; 30%; Fig. 3D), and adenocarcinoma components (20%). Intermingled or transitional areas between these components were noted. On immunohistochemistry, the neuroendocrine components were positive for CD56, synaptophysin, chromogranin, and CK (Fig. 4A). The adenocarcinoma cells were positive for CEA (Fig. 4B), but negative for neuroendocrine markers. The adenocarcinoid components were positive for both CEA (Fig. 4C) and neuroendocrine markers, and the goblet cells were positive for alcian blue staining (amphicrine differentiation). The tumor was found to have invaded through the muscularis propria into the subserosa. The mucosa surrounding the tumor showed marked intestinal metaplasia. One regional lymph node showed a metastatic lesion of poorly differentiated endocrine carcinoma.
Our unique case of synchronous double primary gastric cancer was composed of an adenocarcinoma with micropapillary features and a composite glandular-endocrine cell carcinoma. Micropapillary carcinoma was first reported in the breast by Siriaunkgul and Tavassoli4 in 1993. This tumor has subsequently been described in the stomach.56 Micropapillary carcinoma is rare, and histologically is characterized by small clusters of tumor cells in the clear lacunar spaces simulating lymphatic or vascular channels, with a high metastatic potential to regional lymph nodes.
The distinctive inverted structure of the pseudopapillary clusters lying within clear empty spaces has been attributed to inversion of the cell polarity.78 This reversed arrangement, the so called “inside out growth pattern,” could be confirmed by detection of the inversion of the apical membranous staining pattern for MUC-1 or EMA.
MUC-1 is a glycoprotein normally expressed in the apical membrane of the normal glandular epithelium of some secretory organs, such as the salivary glands, breast, and lung. It has been known to play an important role in lumen formation, and generally inhibits interaction between cell and stroma in the detachment of cells from the stroma.8
The proportion of micropapillary component required for the diagnosis of micropapillary carcinoma has not yet been decided in the above-listed organs. Micropapillary carcinoma may occupy the entire lesion or occur focally within more typical carcinomas in the organs discussed above. However, the recognition of a micropapillary carcinoma component is important, since the presence of this component has been associated with poor prognosis regardless of the amount of tumor cells in other organs.
Micropapillary carcinoma should be differentiated from ordinary adenocarcinoma with pseudomicropapillary features due to extensive lympho-vascular tumor invasion or processing artifacts. However, in our case, the lining cells of most of the empty and clear spaces around micropapillary tumor cells were negative for factor VIII-related antigen, D2-40, and CD34, and the tumor tufts revealed reversed apical membranous immunoreactive patterns for MUC-1 and EMA.
Composite (mixed) glandular-endocrine cell carcinoma is recognized as a special type of gastric tumor, composed of ordinary adenocarcinoma and neuroendocrine tumors. Although a confusing variety of names has been applied to such tumors, a simple nomenclature has been proposed by Lewin9 in which the neoplasms are classified into three groups: (1) mixed tumors with admixed glandular and endocrine elements, with each component comprising at least one third of the tumor; (2) amphicrine tumors with glandular and endocrine differentiation in the same cell; and (3) collision tumors with juxtaposition of these two elements without admixture. Yang and Rotterdam10 reviewed 20 reported cases of mixed (composite) glandular-endocrine cell carcinoma of the stomach, which affected adults aged 32 to 74 years (mean, 52.5 years) and had a male:female ratio of 1.3:1.0. The cancer was located with almost equal frequency in the gastric body and antrum and had a poor prognosis, similar to that of advanced ordinary gastric carcinoma.10
In our case, the patient was alive and disease-free 18 months after surgery. Postoperatively, adjuvant chemotherapy was administered for 6 months, consisting of cisplatin and etoposide. However, 24 months postoperatively, a single metastatic lesion was identified in the brain. The metastatic brain tumor was excised by a neurosurgeon in May 2008; since then recurrent metastatic brain tumor has been excised twice. The metastatic brain tumor was consistent with metastatic neuroendocrine carcinoma, and the patient expired in April 2013.
In this case, the presence of intimate admixed or transitional areas between these components and an adenocarcinoid component revealing amphicrine differentiation support the idea that this tumor might originate from a multipotential precursor cell capable of differentiating along the lines of both an adenocarcinoma and neuroendocrine carcinoma.
In summary, we report a rare double primary gastric cancer consisting of an adenocarcinoma with micropapillary features occurring concurrently with a composite glandular-endocrine cell carcinoma. This case is unique, due to the peculiar histologic features of the micropapillary pattern and the spectrum of adenocarcinoma-adenocarcinoid-neuroendocrine carcinoma of the synchronous composite tumor.
Figures and Tables
Fig. 1
A subtotal gastrectomy specimen showing two well defined tumors. An ulceroinfiltrative tumor (A; 3.5×3.0×0.5 cm) present in the posterior wall of the antrum. The other tumor in the anterior wall of the antrum (B; 3.0×2.8×0.8 cm).
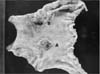
Fig. 2
(A, B) The larger tumor was composed of an invasive micropapillary carcinoma component admixed with moderately differentiated adenocarcinoma components (H&E; A: ×40, B: ×100). (C) MUC-1 expression at the stromal edges of small clusters of the micropapillary carcinoma (immunohistochemistry stain, ×200). (D) Negative D2-40 expression in the lining epithelium of the empty space of the tumor (immunohistochemistry stain, ×200).
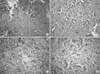
Fig. 3
(A, B) An intermixed area of the adenocarcinoma and goblet cell carcinoid (H&E, ×200). (C) Neuroendocrine cell carcinoma (right side) components adjacent to the adenocarcinoma components (H&E, ×100). (D) Goblet cell or tubular carcinoid (H&E, ×100).

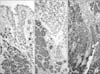
Fig. 4
(A) Chromogranin as expressed in the adenocarcinoid components, but not in the adenocarcinoma cells. (B) Carcinoembryonic antigen (CEA) staining positive in the adenocarcinoma, but negative in the neuroendocrine components. (C) Adenocarcinoid cells were diffusely positive for CEA, but intermixed neuroendocrine carcinoma cells were negative (A~C: immunohistochemistry stain, ×200).
References
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.
2. Bae JS, Lee JH, Ryu KW, Kim YW, Bae JM. Characteristics of synchronous cancers in gastric cancer patients. Cancer Res Treat. 2006; 38:25–29.
3. Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, et al. Incidence, diagnosis and significance of multiple gastric cancer. Br J Surg. 1995; 82:1540–1543.
4. Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993; 6:660–662.
5. Lee JH, Kim JH, Choi JW, Kim YS. The presence of a micropapillary component predicts aggressive behaviour in early and advanced gastric adenocarcinomas. Pathology. 2010; 42:560–563.
6. Eom DW, Kang GH, Han SH, Cheon GJ, Han KH, Oh HS, et al. Gastric micropapillary carcinoma: a distinct subtype with a significantly worse prognosis in TNM stages I and II. Am J Surg Pathol. 2011; 35:84–91.
7. Sakamoto K, Watanabe M, De La, Honda H, Ise H, Mitsui K, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology. 2005; 47:479–484.
8. Nassar H, Pansare V, Zhang H, Che M, Sakr W, Ali-Fehmi R, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004; 17:1045–1050.
9. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987; 11:Suppl 1. 71–86.
10. Yang GC, Rotterdam H. Mixed (composite) glandular-endocrine cell carcinoma of the stomach. Report of a case and review of literature. Am J Surg Pathol. 1991; 15:592–598.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download