Abstract
Purpose
Transducer-like enhancer of split 1 (TLE1) is a member of the Groucho/TLE family of transcriptional co-repressors that regulate the transcriptional activity of numerous genes. TLE1 is involved in the tumorigenesis of various tumors. We investigated the prognostic significance of TLE1 expression and its association with clinicopathological parameters in gastric cancer (GC) patients.
Materials and Methods
Immunohistochemical analysis of six tissue microarrays was performed to examine TLE1 expression using 291 surgically resected GC specimens from the Soonchunhyang University Cheonan Hospital between July 2006 and December 2009.
Results
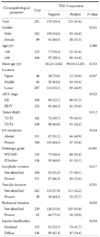
In the non-neoplastic gastric mucosa, TLE1 expression was negative. In GC, 121 patients (41.6%) were positive for TLE1. The expression of TLE1 was significantly associated with male gender (P=0.021), less frequent lymphatic (P=0.017) or perineural invasion (P=0.029), intestinal type according to the Lauren classification (P=0.024), good histologic grade (P<0.001), early pathologic T-stage (P=0.012), and early American Joint Committee on Cancer stage (P=0.022). In the Kaplan-Meier analysis, the TLE1 expression was significantly associated with longer disease-free (P=0.022) and overall (P=0.001) survival rates.
Gastric cancer (GC) is one of the most common types of malignancy and is the third most frequent cause of cancer-related deaths worldwide.1 Recently, several anticancer drugs and surgical techniques have been developed for the treatment of GC; however, the prognosis of patients with advanced GC remains poor. Therefore, identification of new molecular biomarkers that are associated with diagnosis and/or prognosis is of utmost clinical importance.
Transducer-like enhancer of split 1 (TLE1) is a member of the Groucho/TLE family of transcriptional co-repressors and regulates the transcriptional activity of various genes.2 Specifically, TLE1 suppresses E-cadherin, reducing the translation of WNT genes and inhibiting nuclear factor-kappa B regulated gene expression.34 TLE1 is also known to be involved in the regulation of neurogenesis as well as several developmental processes.56
Over-expression of the TLE1 gene was identified in synovial sarcomas by DNA microarray.78 Additionally, using immunohistochemistry (IHC), several studies have reported TLE1 to be a specific diagnostic marker in synovial sarcomas.910 These studies support the diagnostic utility of TLE1 expression in synovial sarcomas. Other studies have demonstrated non-specific TLE1 expression in non-synovial sarcomas, including neurofibromas, schwannomas, malignant peripheral nerve sheath tumors, solitary fibrous tumors, and mesotheliomas.111213 TLE1 expression has also been demonstrated in various cell types of normal tissues, including basal keratinocytes and adipocytes, as well as perineural, endothelial, and mesothelial cells.111213 In hematological malignancies, the TLE1 gene is inactivated and acts as a tumor suppressor in myeloid leukemia by inhibiting cell proliferation and colony formation.1415 Zhang et al.16 reported a decrease in TLE1 expression in hepatocellular carcinomas compared to that in their adjacent non-cancerous tissues. This may suggest that the TLE1 gene plays an important role in liver tumor suppression. On the other hand, TLE1 has been shown to be selectively over-expressed in invasive breast tumors relative to non-invasive ductal carcinomas in situ and normal mammary epithelial tissues.17 Yao et al.18 suggested that TLE1 improves epithelial-to-mesenchymal transition in lung cancer cells through the suppression of E-cadherin and has the potential to regulate the aggressiveness of lung cancers.
Recent studies have demonstrated the variable functions of TLE1 in a number of malignancies. However, to our knowledge, TLE1 expression has not yet been evaluated in gastric adenocarcinomas. In the present study, we investigated TLE1 expression in surgically resected GC patients using IHC. The aim of our investigation was to examine the prognostic significance of TLE1 expression and to determine its association with clinicopathological parameters in GC patients.
We retrospectively analyzed data from 291 patients who underwent surgical resection for GC at the Soonchunhyang University Cheonan Hospital (Cheonan, Korea) between July 2006 and December 2009. Patient medical records were reviewed for clinicopathological information, including age, gender, tumor location, TNM stage, tumor differentiation, presence of lymphatic, vascular, or perineural invasion, and Lauren classification. Patient survival data was obtained by reviewing the patients' medical records or through the death registry offices. All cases were histopathologically re-examined by two independent pathologists (JH Lee and KJ Kim) to confirm the diagnosis and other pathological features. Tumor stages and grades were re-classified according to the seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual. We excluded patients who presented with other critical medical conditions or had received neoadjuvant chemotherapy and cases where tissue blocks were unavailable.
This study was approved by the Institutional Review Board at Soonchunhyang University (SCHCA 2015-11-026).
Tissue microarrays (TMAs) were constructed by reviewing hematoxylin and eosin-stained slides and selecting one representative formalin-fixed paraffin-embedded archival block for each case. Tissue cores (2-mm thick) were extracted from individual formalin-fixed paraffin-embedded blocks (donor blocks) and rearranged into recipient paraffin blocks (TMA blocks) by using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea). In addition, normal gastric mucosa specimens were included in 26 cases by using the same procedure. One section from the TMA block was stained with hematoxylin and eosin for tissue confirmation.
TLE1 expression was analyzed by IHC. Details of the materials and methods used in this study have been reported elsewhere and will be described briefly here. Tissue sections (4 µm thick) extracted from the TMA blocks were transferred to poly-L-lysinecoated glass slides and incubated in a dry oven at 60℃ for 1 hour. These sections were then de-waxed in xylene (three changes), rehydrated in a graded series of decreasing ethanol concentrations, and rinsed in Tris-buffered saline solution (pH 7.4). Endogenous peroxidase activity was inactivated with 5% hydrogen peroxide in methanol at 37℃ for 15 minutes. For TLE1 staining, antigen retrieval was performed using a microwave treatment in an epitope retrieval solution (pH 6.0) for 20 minutes. The tissue sections were incubated with a mouse monoclonal antibody against TLE1 (1:50 dilution, 1F5; Cell Marque Corp., Rocklin, CA, USA) in a humidified chamber at 4℃ for 16 hours. A secondary antibody was then applied using a Bond Polymer Refine Detection kit (Leica, Wetzlar, Germany). Diaminobenzidine was used as the chromogen, and the tissue sections were counterstained using Mayer's hematoxylin solution. Positive controls, consisting of cases with known reactivity for the antibody, and negative controls, which were obtained by omitting the primary antibody, were also included.
IHC staining was independently evaluated by JH Lee and KJ Kim, and in the rare instances where there was a discrepancy in their judgment, the two investigators reviewed the slides together using a multi-head microscope. Semi-quantitative IHC scores were assigned that included an assessment of both intensity and the extent of staining. The intensity of staining was scored on a scale of 0 to 3, corresponding to negative, weak, moderate, and strong positivity, respectively (Fig. 1). The extent of staining was also scored on a scale of 0 to 3, according to the percentages of cells (0, ≤10, >10 and ≤50, or >50%, respectively) that stained positively for each protein. The product of the intensity and extent scores was used to denote the final score (i.e., 0, 1, 2, 3, 4, 6, or 9). Tissues with a final score of ≥2 were considered positive for TLE1 expression. Similar semi-quantitative scoring systems have been successfully used for other TMA assessments.19
Statistical analyses were conducted using SPSS for Windows software ver. 19.0 (IBM Co., Armonk, NY, USA). Associations between TLE1 expression and clinicopathological parameters of the patients were assessed using the Pearson's chi-squared and Fisher's exact test. Disease-free survival (DFS) was defined as the duration in months from the date of surgery to the date of death, tumor recurrence, or last follow-up. Overall survival (OS) was defined as the duration from the date of surgery to the date of death or last follow-up. DFS and OS rates in relation to TLE1 expression were calculated using the Kaplan-Meier method. To assess differences between Kaplan-Meier curves, a log-rank test was performed. Statistical significance was defined as a P-value below 0.05.
Of 291 GC patients included in this study, 202 were male and 89 were female. The age at diagnosis (mean±standard deviation) was 59.9±12.480 years (range, 25 to 85 years). The cohort comprised of 115 early and 176 advanced GC cases. Histologically, there were 155 tubular carcinomas, 106 poorly cohesive carcinomas that included signet ring cell carcinomas, 9 mucinous adenocarcinomas, 2 papillary carcinomas, 16 mixed carcinomas, and 3 unclassified carcinomas. In total, there were 111 stage I, 58 stage II, 112 stage III, and 10 stage IV tumors. At the point of diagnosis, 160 cases showed signs of lymph node metastasis and 10 cases showed signs of distant metastasis.
In the non-neoplastic gastric mucosa, TLE1 expression was negative. In GC, 121 patients (41.6%) were positive for TLE1 (Table 1). Table 2 summarizes TLE1 expression and clinicopathological parameters of the cohort. TLE1 expression was significantly associated with male gender (P=0.021), less frequent lymphatic (P=0.017) or perineural (P=0.029) invasion, intestinal type according to the Lauren classification (P=0.024), good histologic grade (P<0.001), early pathologic T-stage (P=0.012), and early AJCC stage (P=0.022). TLE1 expression also showed GCs to be located in the lower third of the stomach, although this did not reach statistical significance (P=0.057). TLE1 expression was not found to be associated with age or vascular invasion.
The follow-up duration across the patient cohort ranged from 1 to 95 months (median duration of DFS, 56 months; OS, 65 months). During follow-up, four patients (1.4%) had local recurrence and 46 patients (15.8%) had distant metastases. In addition, 68 patients (23.4%) died. According to the Kaplan-Meier analysis, TLE1 expression was significantly associated with longer DFS (P=0.022) and OS rates (P=0.001) (Fig. 2). Additionally, male gender, lymphatic or perineural invasion, poor histologic grade, more advanced pathologic T/N stages, and more advanced AJCC stages were significantly associated with shorter DFS (P<0.05) and OS rates (P<0.05). In the multivariate Cox regression analysis, TLE1 expression was not significantly associated with survival (data not shown).
The transcriptional co-repressor TLE1 does not bind directly to DNA, but instead interacts with other DNA-binding transcription factors to form large multi-protein complexes that are recruited to the target gene.20 For example, TLE proteins are involved in the Wnt/β-catenin signaling pathway. These proteins bind to the transcription factor, lymphoid enhancer-binding factor 1 that displaces the Wnt activator, β-catenin. Consequently, there is a reduction in the translation of WNT genes.3 Recently, several studies have demonstrated the oncogenic effect of TLE1. TLE1 has been shown to inhibit the caspase-independent cell death pathway induced by the Bcl2-inhibitor of transcription 1 in various malignant cells.21 Allen et al.22 demonstrated that TLE1 positively regulates erb-B2 receptor tyrosine kinase 1 and 2 signaling and is over-expressed in a subset of human non-small cell carcinomas. TLE1 also potentiates epithelial-to-mesenchymal transition, partly through the suppression of E-cadherin, by recruiting histone deacetylase activity to the E-cadherin promoter in lung cancer cells.18
In the present study, we investigated TLE1 expression in normal gastric mucosa and GC tissues. TLE1 was not expressed in normal gastric mucosa. However, TLE1 expression was detected in 41.6% of GC patients. These findings were similar to those reported previously in lung and breast cancers.1722 Brunquell et al.17 demonstrated an up-regulation of TLE1 immuno-reactivity in invasive breast carcinoma tissues compared to normal/ductal carcinoma in situ sub-groups, and TLE1 expression was detected in 67% of invasive breast carcinomas. TLE1 suppression may contribute to the sensitivity of normal epithelial cells to anoikis. However, loss of matrix interaction results in the up-regulation of TLE1 expression in breast cancer cells. This confers protection against anoikis and promotes anchorage-independent growth partly through the inhibition of the Bcl2 inhibitor of transcription 1 anoikis pathway. Allen et al.22 demonstrated TLE1 expression in 11% of squamous cell carcinomas and 20% of adenocarcinomas in human lung tumors and concluded that TLE1 was involved in increasing erb-B2 receptor tyrosine kinase 1 and 2 signaling. Conversely, in hepatocellular carcinomas, TLE1 expression was shown to be reduced compared to that in adjacent non-cancerous tissues.16 Zhang et al.16 suggested that TLE1 expression could influence tumor suppression in hepatocarcinogenesis through the inactivation of the nuclear factor-kappa B pathway. In the present study, we concluded that TLE1 might have an oncogenic role in GC.
We also demonstrated a significant association between TLE1 expression and a number of informative prognostic clinicopathological parameters. For example, TLE1 expression was significantly associated with early pathologic T/N and AJCC stages, and good histologic grades. TLE1 expression was also associated with less frequent lymphatic or perineural invasion and intestinal type according to Lauren classification. Univariate analysis of DFS and OS rates showed TLE1 expression was strongly associated with longer survival, but this association was not validated by multivariate analysis. Apart from those mentioned, the mechanism of TLE1 action in human cancers is not yet known. Different functional mechanisms of TLE1 have not yet been found that seem to lead to this result. The present study shows that TLE1 expression is a good indicator of prognosis in GCs although it is not the sole prognostic factor. Only a few studies have investigated the expression of TLE1 in human cancer tissues.161722 To our knowledge, no studies have demonstrated an association between TLE1 expression and the clinicopathological parameters and patient outcomes in carcinomas. The present study is the first to assess TLE1 expression in GC patients and to determine its prognostic significance. Additional studies are needed to further evaluate the relationship between TLE1 expression and prognostic factors including various clinicopathological parameters and patient survival rates in different carcinomas, and to identify alterative mechanism(s) for the prognostic effect of TLE1.
In conclusion, we are the first to demonstrate the presence of TLE1 expression in 41.6% of GCs and absence in normal gastric mucosa. This suggests that TLE1 has an oncogenic effect in gastric carcinogenesis. However, expression of TLE1 is significantly correlated with better prognostic factors, including early T/N stages, early AJCC stages, and longer DFS and OS rates. This suggests that TLE1 expression is a good prognostic indicator in GCs. However, further studies are required to understand the mechanism of TLE1 when its expression appears contradictory.
Figures and Tables
Fig. 1
Immunohistochemical (IHC) staining of transducer-like enhancer of split 1 (TLE1) in normal gastric mucosa and gastric cancer (GC). (A) Representative images of TLE1 IHC of normal gastric mucosa (×200) exhibiting a score of 0. (B) Representative images of TLE1 IHC of GCs (×400) exhibiting a score of 1. (C) Representative images of TLE1 IHC of GCs (×400) exhibiting a score of 2. (D) Representative images of TLE1 IHC of GCs (×400) exhibiting a score of 3.
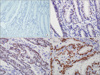
Fig. 2
Kaplan-Meier survival analysis with log-rank test. Transducer-like enhancer of split 1 expression was significantly associated with good overall survival (A) and good disease-free survival (B).

References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Chen G, Courey AJ. Groucho/TLE family proteins and transcriptional repression. Gene. 2000; 249:1–16.
3. Arce L, Pate KT, Waterman ML. Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer. 2009; 9:159.
4. Ghosh HS, Spencer JV, Ng B, McBurney MW, Robbins PD. Sirt1 interacts with transducin-like enhancer of split-1 to inhibit nuclear factor kappaB-mediated transcription. Biochem J. 2007; 408:105–111.
5. Buscarlet M, Hermann R, Lo R, Tang Y, Joachim K, Stifani S. Cofactor-activated phosphorylation is required for inhibition of cortical neuron differentiation by Groucho/TLE1. PLoS One. 2009; 4:e8107.
6. Flowers EB, Poole RJ, Tursun B, Bashllari E, Pe'er I, Hobert O. The Groucho ortholog UNC-37 interacts with the short Groucho-like protein LSY-22 to control developmental decisions in C. elegans. Development. 2010; 137:1799–1805.
7. Allander SV, Illei PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, et al. Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol. 2002; 161:1587–1595.
8. Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N, et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002; 62:5859–5866.
9. Jagdis A, Rubin BP, Tubbs RR, Pacheco M, Nielsen TO. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol. 2009; 33:1743–1751.
10. Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011; 135:839–844.
11. Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009; 22:872–878.
12. Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007; 31:240–246.
13. Matsuyama A, Hisaoka M, Iwasaki M, Iwashita M, Hisanaga S, Hashimoto H. TLE1 expression in malignant mesothelioma. Virchows Arch. 2010; 457:577–583.
14. Fraga MF, Berdasco M, Ballestar E, Ropero S, Lopez-Nieva P, Lopez-Serra L, et al. Epigenetic inactivation of the Groucho homologue gene TLE1 in hematologic malignancies. Cancer Res. 2008; 68:4116–4122.
15. Dayyani F, Wang J, Yeh JR, Ahn EY, Tobey E, Zhang DE, et al. Loss of TLE1 and TLE4 from the del(9q) commonly deleted region in AML cooperates with AML1-ETO to affect myeloid cell proliferation and survival. Blood. 2008; 111:4338–4347.
16. Zhang L, Yang L, Liu X, Chen W, Chang L, Chen L, et al. MicroRNA-657 promotes tumorigenesis in hepatocellular carcinoma by targeting transducin-like enhancer protein 1 through nuclear factor kappa B pathways. Hepatology. 2013; 57:1919–1930.
17. Brunquell C, Biliran H, Jennings S, Ireland SK, Chen R, Ruoslahti E. TLE1 is an anoikis regulator and is downregulated by Bit1 in breast cancer cells. Mol Cancer Res. 2012; 10:1482–1495.
18. Yao X, Ireland SK, Pham T, Temple B, Chen R, Raj MH, et al. TLE1 promotes EMT in A549 lung cancer cells through suppression of E-cadherin. Biochem Biophys Res Commun. 2014; 455:277–284.
19. Lee HW, Kim EH, Oh MH. Clinicopathologic implication of ezrin expression in non-small cell lung cancer. Korean J Pathol. 2012; 46:470–477.
20. Gasperowicz M, Otto F. Mammalian Groucho homologs: redundancy or specificity? J Cell Biochem. 2005; 95:670–687.
21. Jan Y, Matter M, Pai JT, Chen YL, Pilch J, Komatsu M, et al. A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell. 2004; 116:751–762.
22. Allen T, van Tuyl M, Iyengar P, Jothy S, Post M, Tsao MS, et al. Grg1 acts as a lung-specific oncogene in a transgenic mouse model. Cancer Res. 2006; 66:1294–1301.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download