Abstract
In Korea, proximal gastrectomy has recently attracted attention as a better choice of function-preserving surgery for proximal early gastric cancer than total gastrectomy. Of the various strategies to overcome reflux symptoms from remnant stomach, double tract reconstruction not only reduces the incidence of anastomosis-related complications, but is also sufficiently reproducible as a laparoscopic procedure. Catching up with the recent rise of single-port laparoscopic surgeries, we performed a pure single-port laparoscopic proximal gastrectomy with DTR. This procedure was designed by merging the function-preserving concept of proximal gastrectomy with single-port laparoscopic total gastrectomy.
In Korea, proximal gastrectomy (PG) has recently attracted attention as a better choice of function-preserving surgery for proximal early gastric cancer (EGC) than total gastrectomy (TG). Although the remnant stomach after PG may be a source for cancer recurrence, several data have advocated for the oncologic safety of this procedure.123 With regard to conventional PG, the more troublesome issue has been reflux symptoms induced by esophagogastric anastomosis where the esophageal mucosa is exposed to gastric juice. Therefore, various strategies have been designed to reduce reflux from remnant stomach.24567891011121314151617181920212223242526272829303132 Of these procedures, jejunal interposition (JI) and double tract reconstruction (DTR) modify the structure of esophagojejunostomy (EJ), and therefore the jejunum acts as a buffer between esophagus and remnant stomach. However, JI is associated with abdominal discomfort after meals, continuous gastric fullness, and hiccups between meals. These symptoms may result from the interposed segment, which may disturb the passage of food.2533 Additionally, it is difficult to reproduce the complicated structure of JI during a laparoscopic procedure. Conversely, DTR not only reduces the incidence of anastomosis-related complications, but is also sufficiently reproducible as a laparoscopic procedure.34 For these reasons, in Korea, DTR is recently supported by most surgeons who prefer laparoscopic PG for the treatment of proximal EGC. DTR was adopted in an ongoing multi-center prospective study, KLASS05, in which clinical outcomes will be compared between laparoscopic PG and TG for proximal EGC.
Nevertheless, single-port laparoscopic procedures have not been easy to apply in PG with DTR. Even in conventional laparoscopic PG with DTR, EJ embeds a high possibility of anastomotic failure. Therefore, it has been considered impellent to achieve EJ with bearing the poor ergonomics of single-port laparoscopic surgery. Moreover, since DTR results in more complex structures than conventional esophagogastrostomy, some surgeons are concerned about the short-term safety of laparoscopic PG with DTR. Therefore, few surgeons have planned PG with DTR in the setting of single-port laparoscopic surgery.
Recently, for a cT1 case, we performed a single-port laparoscopic proximal gastrectomy (SPPG) with DTR. Our center has previously performed single-port laparoscopic distal gastrectomy (SPDG) for EGC; therefore, the procedures presented in this case report were planned based on our expertise. To the best of our knowledge, this is the first report of SPPG with DTR.
A 40-year-old woman with a poorly differentiated gastric adenocarcinoma diagnosed via gastroscopic biopsy was referred to our center. The tumor was located on the posterior wall of the gastric high body. Computed tomography showed that there was no regional lymph node (LN) involvement or distant metastasis (cT1N0M0). Her body mass index (BMI) was 22.5 kg/m2 and she did not have any comorbid conditions or history of previous abdominal surgery.
We decided to perform a PG with D1+ lymph node dissection (LND) since the patient presented with early-stage disease in the proximal stomach.35 DTR was adopted to avoid the previously described complications associated with conventional esophagogastrostomy.34 In addition, considering her low BMI, single-port laparoscopic surgery was planned. The details of our procedure were as follows:
In the operating room, the patient was placed on the bed with both legs abducted under general anesthesia. The bed was adjusted to create a reverse Trendelenburg position for the patient. The operator stood between the patient's legs. The scopist was positioned on the left side of the patient.
A commercial 4-lumen single-port trocar (Gloveport®; Nelis, Bucheon, Korea) was inserted through a transumbilical incision using Hasson's method.36 The Gloveport trocar system consists of a self retractor that covers a 25 mm incision and four channels (one 12 mm channel and three 5 mm channels). After a pneumoperitoneum was formed with carbon dioxide at a pressure of 15 mmHg, a flexible scope was inserted through a channel of the umbilical port. The falciform ligament and the left lobe of the liver were raised in the cephald direction by combined suture retraction.37
One surgeon performed intraoperative endoscopy for tumor localization while another surgeon carefully manipulated the stomach in the laparoscopic field. The endoscopic view was used to compare the location of preoperatively applied clips to that of the mucosal protrusion formed by laparoscopic manipulation. Both surgeons compared the endoscopic and laparoscopic views, and determined the location of the tumor in the laparoscopic view.
D1+ LND was performed based upon the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3).35 A commercial prebent instrument (Olympus Medical System Corp., Tokyo, Japan) and Harmonic Scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) were used to facilitate LND.
1) Partial omentectomy (Fig. 1A) began with the division of the greater omentum more than 4 cm from the gastroepiploic arcade to include LN station No. 4d. The left gastroepiploic and short gastric vessels were ligated and divided to dissect LN station No. 4sb and 4sa.
2) After this procedure, the direction of LND was changed toward the right side of the stomach. The greater omentum was divided to mobilize the distal stomach, but the right gastroepiploic vessels were not divided to preserve the blood supply to the distal stomach.
3) The lesser omentum was then divided to expose LN station No. 8a. While preserving the right gastric vessels, LN station No. 8a was dissected until the common hepatic artery was exposed (Fig. 1B).
4) After the lesser and greater curvatures of the stomach were cleared by ligation and division of the left gastric and gastroepiploic arcades, the stomach was divided 2 cm distal to the gastric lesion using linear stapler (Echelon Flex™ GST system; Ethicon Endo-Surgery Inc.) (Fig. 1C).
5) Suprapancreatic LND was performed to clear LN station No. 7, 9, and 11p (Fig. 1D)
6) After the left gastric vessels were ligated and divided, the esophagus was dissected for division. Esophageal division was also accomplished with linear stapler (Fig. 1E).
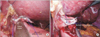
1) To keep stable traction of the esophageal stump, two tagging sutures were placed at both edges of the stapled line. Then, EJ was performed using linear stapler (Fig. 2A). The common entry hole for the anvils was closed with barbed suture material (V-LocTM; Medtronics, Minneapolis, MN, USA) (Fig. 2B).
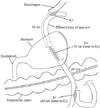
2) Gastrojejunostomy (GJ) and jejunojejunostomy (JJ) were also completed with linear stapler and barbed suture material. GJ was made 15 cm distal to the EJ, and JJ was made 20 cm distal to the GJ (Fig. 3).
A closed suction drainage tube was inserted through the umbilical incision.
The total operation time was 350 minutes. The patient start drinking water on the postoperative day eighth, after we confirmed the results of esophagography. A semibland diet was provided on the postoperative day ninth. The patient was discharged with no complications on the postoperative day 10th. The final pathology report revealed that the tumor had invaded the submucosa. One LN metastasis was found among the 22 LNs that had been dissected (pT1bN1, stage IB; according to the American Joint Committee on Cancer 7th edition).38 Lymphovascular invasion was also noted
The patient came for a follow-up appointment on the postoperative day 20th. We did not find any wound infections (Fig. 4) or dietary problems. Since then, the patient has taken oral 5-FU (fluorouracil) as adjuvant chemotherapy. We did not find any evidence of recurrence for 10 months after the operation. Furthermore, the patient has not suffered from any reflux symptoms. Her total weight loss after surgery was 2.65 kg
Few surgeons have adopted single-port laparoscopic surgery for gastric cancer, as some technical complexity is embedded in gastric cancer surgery. It is technically demanding to perform a sufficient LND with straight instruments inserted through only one trocar, since the stomach is supplied and drained by several vessels that run in diverse directions. Such limited conditions also exist in the process of reconstruction. Moreover, many surgeons are concerned about the level of difficulty involved with correcting unexpected accidents during a single-port laparoscopic surgery.
Despite these limitations, several surgeons have reported clinical outcomes of SPDG for EGC.39404142434445 Although the long-term oncologic outcomes have not been investigated, a favorable prognosis is expected based on several reports showing the number of harvested LNs after SPDG for EGC.424446 In addition, according to a recent comparative study, the short-term clinical outcomes of SPDG were not inferior to those of multi-port laparoscopic distal gastrectomy for EGC.42 Moreover, Ahn et al.4748 reported the technical feasibility of performing EJ during single-port laparoscopic total gastrectomy (SPTG) for proximal EGC. Since EJ has been regarded as a challenging procedure even in conventional laparoscopic surgery, SPTG is a noteworthy achievement in the era of minimally invasive surgery.
However, it is necessary to consider the reasons why SPTG is not widely accepted for gastric cancer surgery. SPTG is indicated for a limited number of cases, as we consider the following issues; First, the current ergonomics of single-port laparoscopic surgery are too limited to guarantee consistent quality of D2 LND, and therefore SPTG is not a rational strategy for advanced gastric cancer. Second, although SPTG may be performed for proximal EGC, most surgeons hesitate to perform a TG for cT1 lesion.
Considering these aspects, we planned SPPG with DTR for proximal EGC. This procedure was designed by merging the function-preserving concept of PG with SPTG. For the last decades, PG has been performed as a function-preserving surgery for proximal EGC in our institute. After much trial and error, we recently adopted DTR as a reconstruction method after PG. The most important reason why this method was adopted was its effectiveness in reducing reflux symptoms.34 In addition, PG with DTR was familiar to surgeons who have performed TG, since EJ and JJ are commonly included in both procedures. Similarly, we have also gained experience with laparoscopic PG with DTR due to our previous experience with laparoscopic TG.
Moreover, since we had also experience with SPDG for EGC, SPPG with DTR could be designed based on our accumulated expertise. LND was smoothly completed in SPPG because we had previously performed D1+ LND during SPDG for EGC. Even though LND of No. 4sa and 11p were newly required in SPPG, these procedures were securely performed based on previous practices. Additionally, as we had been operating with linear staplers in SPDG, most aspects of the reconstruction were familiar. GJ, a unique anastomosis of SPPG with DTR, was also achieved in a similar manner to the reconstruction of SPDG with Billroth II.
However, some new issues should be considered when performing EJ. The first issue was the direction of efferent loop. According to Ahn et al.,4748 since they use the modified semi-loop method during SPTG, the efferent loop of EJ proceeded toward the left side of abdominal cavity. On the other hand, during SPPG with DTR, efferent loop should be kept to the right until GJ is made (Fig. 3). Although the modified semi-loop method is a reliable technique for EJ, jejunum should be twisted to meet the remnant stomach in the opposite location. Therefore, in order to send the efferent loop toward the remnant stomach without twisting the jejunum, we introduced the overlap method.4950
Another issue noted during EJ was closing the common entry hole, into which each anvil of linear stapler had been inserted for anastomosis (Fig. 2B). In this process, we adopted 'hand-sewing' in order to minimize the risk of anastomotic stenosis. According to the original report of overlap method, linear stapler was not used in closing the common entry hole for the same reason.49 A great deal of concentration was required to close the common entry hole by hand-sewing because the axis of the laparoscopic needling was nearly parallel to the suture direction. Moreover, despite such poor ergonomics, every needling should pass precisely through each layer of esophagus and jejunum to make a stable anastomosis. This difficulty may be resolved if controllable articulation is introduced in the laparoscopic instrument. A recently released 'articulating instrument' (SILS™ Hand instrument; Medtronics) could not lessen the adversity of this procedure; therefore, the further development of laparoscopic instrument is necessary for generalization of SPPG or SPTG. This issue is also related to the potential of robotic surgery. Although the present status of robotic surgery does not realize a pure single-port laparoscopic surgery for gastric cancer, the technical difficulties we encountered during SPPG or SPTG may provide clues as to how robotic surgery could be developed.
In conclusion, despite some technical challenges caused by the poor ergonomics, SPPG with DTR resulted in acceptable short-term outcomes. In addition, it is inspiring that total weight loss was less than 3 kg, although further follow-up is necessary to evaluate the nutritional effect of remnant stomach. Moreover, the patient has undergone no reflux symptom for 10 months after the operation. Whereas only the technical achievement has been emphasized in the previously issued single-port laparoscopic procedures,39404142434445464748 SPPG with DTR provided not only a cosmetic benefit but also good quality of life for the patient. In the era of function-preserving surgery, such advantages may correspond with the future perspective of single-port laparoscopic surgery for EGC.
Figures and Tables
Fig. 1
The procedures during lymph node dissection. (A) Opening of the lesser sac. (B) Lymph node station No. 8a (RGA = right gastric artery; CHA = common hepatic artery). (C) Division of stomach with linear staper. (D) Lymph node station No. 11p. (E) Division of esophagus with linear stapler.
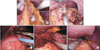
Fig. 2
The details of esophgojejunostomy. (A) Esophagojejunostomy with linear stapler. (B) Closure of common entry hole with a barbed suture material.
References
1. Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998; 123:127–130.
2. Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg. 2013; 37:558–564.
3. Zeng C, Huang L, Chen L, Huang H, Zheng Y, Chi L. Comparison of short- and long-term efficacy of three procedures in postoperative digestive tract reconstruction for upper gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2014; 17:444–448.
4. Kong SH, Kim JW, Lee HJ, Kim WH, Lee KU, Yang HK. Reverse double-stapling end-to-end esophagogastrostomy in proximal gastrectomy. Dig Surg. 2010; 27:170–174.
5. Zhao P, Xiao SM, Tang LC, Ding Z, Zhou X, Chen XD. Proximal gastrectomy with jejunal interposition and TGRY anastomosis for proximal gastric cancer. World J Gastroenterol. 2014; 20:8268–8273.
6. Kikuchi S, Nemoto Y, Katada N, Sakuramoto S, Kobayashi N, Shimao H, et al. Results of follow-up endoscopy in patients who underwent proximal gastrectomy with jejunal interposition for gastric cancer. Hepatogastroenterology. 2007; 54:304–307.
7. Ohyama S, Tokunaga M, Hiki N, Fukunaga T, Fujisaki J, Seto Y, et al. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer. 2009; 12:88–94.
8. Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T, et al. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg. 2008; 32:1473–1477.
9. Iwata T, Kurita N, Ikemoto T, Nishioka M, Andoh T, Shimada M. Evaluation of reconstruction after proximal gastrectomy: prospective comparative study of jejunal interposition and jejunal pouch interposition. Hepatogastroenterology. 2006; 53:301–303.
10. Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014; 38:1100–1106.
11. Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005; 29:1592–1599.
12. Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015; 18:407–416.
13. Nomura E, Lee SW, Tokuhara T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and type of reconstruction following open and laparoscopic proximal gastrectomy for gastric cancer. Hepatogastroenterology. 2012; 59:1677–1681.
14. Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg. 2013; 398:697–701.
15. Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: short- and long-term consequences. Am J Surg. 2012; 204:203–209.
16. Hirai T, Matsumoto H, Iki K, Hirabayashi Y, Kawabe Y, Ikeda M, et al. Lower esophageal sphincter- and vagus-preserving proximal partial gastrectomy for early cancer of the gastric cardia. Surg Today. 2006; 36:874–878.
17. Adachi Y, Katsuta T, Aramaki M, Morimoto A, Shiraishi N, Kitano S. Proximal gastrectomy and gastric tube reconstruction for early cancer of the gastric cardia. Dig Surg. 1999; 16:468–470.
18. Ronellenfitsch U, Najmeh S, Andalib A, Perera RM, Rousseau MC, Mulder DS, et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015; 22:772–779.
19. Kondoh Y, Ishii A, Ishizu K, Hanashi T, Okamoto Y, Morita M, et al. Esophagogastrostomy before proximal gastrectomy in patients with early gastric cancers in the upper third of the stomach. Tokai J Exp Clin Med. 2006; 31:146–149.
20. Ishigami S, Uenosono Y, Arigami T, Kurahara H, Okumura H, Matsumoto M, et al. Novel fundoplication for esophagogastrostomy after proximal gastrectomy. Hepatogastroenterology. 2013; 60:1814–1816.
21. Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010; 97:558–562.
22. Adachi Y, Inoue T, Hagino Y, Shiraishi N, Shimoda K, Kitano S. Surgical results of proximal gastrectomy for early-stage gastric cancer: jejunal interposition and gastric tube reconstruction. Gastric Cancer. 1999; 2:40–45.
23. Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, et al. Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery. 2014; 156:57–63.
24. Takagawa R, Kunisaki C, Kimura J, Makino H, Kosaka T, Ono HA, et al. A pilot study comparing jejunal pouch and jejunal interposition reconstruction after proximal gastrectomy. Dig Surg. 2010; 27:502–508.
25. Tokunaga M, Hiki N, Ohyama S, Nunobe S, Miki A, Fukunaga T, et al. Effects of reconstruction methods on a patient's quality of life after a proximal gastrectomy: subjective symptoms evaluation using questionnaire survey. Langenbecks Arch Surg. 2009; 394:637–641.
26. Ichikawa D, Ueshima Y, Shirono K, Kan K, Shioaki Y, Lee CJ, et al. Esophagogastrostomy reconstruction after limited proximal gastrectomy. Hepatogastroenterology. 2001; 48:1797–1801.
27. Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002; 26:1150–1154.
28. Tomita R, Fujisaki S, Tanjoh K, Fukuzawa M. A novel operative technique on proximal gastrectomy reconstructed by interposition of a jejunal J pouch with preservation of the vagal nerve and lower esophageal sphincter. Hepatogastroenterology. 2001; 48:1186–1191.
29. Yabusaki H, Nashimoto A, Matsuki A, Aizawa M. Evaluation of jejunal pouch interposition after proximal gastrectomy for early gastric cancer in the upper third of the stomach. Hepatogastroenterology. 2012; 59:2032–2036.
30. Kobayashi M, Araki K, Okamoto K, Okabayashi T, Akimori T, Sugimoto T. Anti-reflux pouch-esophagostomy after proximal gastrectomy with jejunal pouch interposition reconstruction. Hepatogastroenterology. 2007; 54:116–118.
31. Hoshikawa T, Denno R, Ura H, Yamaguchi K, Hirata K. Proximal gastrectomy and jejunal pouch interposition: evaluation of postoperative symptoms and gastrointestinal hormone secretion. Oncol Rep. 2001; 8:1293–1299.
32. Zhao Q, Li Y, Guo W, Zhang Z, Ma Z, Jiao Z. Clinical application of modified double tracks anastomosis in proximal gastrectomy. Am Surg. 2011; 77:1593–1599.
33. Hiki N, Nunobe S, Kubota T, Jiang X. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol. 2013; 20:2683–2692.
34. Ahn SH, Jung DH, Son SY, Lee CM, Park DJ, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. 2014; 17:562–570.
35. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
36. Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971; 110:886–887.
37. Shabbir A, Lee JH, Lee MS, Park DJ, Kim HH. Combined suture retraction of the falciform ligament and the left lobe of the liver during laparoscopic total gastrectomy. Surg Endosc. 2010; 24:3237–3240.
38. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17:3077–3079.
39. Omori T, Tanaka K, Tori M, Ueshima S, Akamatsu H, Nishida T. Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc. 2012; 26:1490–1494.
40. Park DJ, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012; 22:e214–e216.
41. Ahn SH, Son SY, Lee CM, Jung DH, Park do J, Kim HH. Intracorporeal uncut Roux-en-Y gastrojejunostomy reconstruction in pure single-incision laparoscopic distal gastrectomy for early gastric cancer: unaided stapling closure. J Am Coll Surg. 2014; 218:e17–e21.
42. Ahn SH, Son SY, Jung DH, Park do J, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg. 2014; 219:933–943.
43. Ahn SH, Jung DH, Son SY, Park do J, Kim HH. Pure single-incision laparoscopic D2 lymphadenectomy for gastric cancer: a novel approach to 11p lymph node dissection (midpancreas mobilization). Ann Surg Treat Res. 2014; 87:279–283.
44. Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, et al. Comparison of single-port and reduced-port totally laparoscopic distal gastrectomy for patients with early gastric cancer. Surg Endosc. 2015; [In print]. DOI: 10.1007/s00464-015-4706-8.
45. Kim SM, Lee SH, Ha MH, Seo JE, Kim JE, Choi MG, et al. Techniques of the single-port totally laparoscopic distal gastrectomy. Ann Surg Oncol. 2015; 22:Suppl 3. S341.
46. Suh YS, Park JH, Kim TH, Huh YJ, Son YG, Yang JY, et al. Unaided stapling technique for pure single-incision distal gastrectomy in early gastric cancer: unaided delta-shaped anastomosis and uncut Roux-en-Y anastomosis. J Gastric Cancer. 2015; 15:105–112.
47. Ahn SH, Son SY, Jung DH, Park YS, Shin DJ, Park DJ, et al. Solo intracorporeal esophagojejunostomy reconstruction using a laparoscopic scope holder in single-port laparoscopic total gastrectomy for early gastric cancer. J Gastric Cancer. 2015; 15:132–138.
48. Ahn SH, Park DJ, Son SY, Lee CM, Kim HH. Single-incision laparoscopic total gastrectomy with D1+beta lymph node dissection for proximal early gastric cancer. Gastric Cancer. 2014; 17:392–396.
49. Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010; 211:e25–e29.
50. Tsujimoto H, Uyama I, Yaguchi Y, Kumano I, Takahata R, Matsumoto Y, et al. Outcome of overlap anastomosis using a linear stapler after laparoscopic total and proximal gastrectomy. Langenbecks Arch Surg. 2012; 397:833–840.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download