Abstract
Hypercalcemia of malignancy due to metastatic gastric adenocarcinoma is extremely rare; in fact, to the best of our knowledge, only three case reports of hypercalcemia associated with metastatic gastric adenocarcinoma have been published in the literature to date. Herein, we report a rare case involving a 61-year-old African-American female who had hypercalcemia at initial presentation and who was later diagnosed with poorly differentiated gastric adenocarcinoma with extensive liver metastases, without bone involvement. She was found to have elevated parathyroid hormone-related peptide and normal parathyroid hormone levels. Despite aggressive treatment, she died within a few months of diagnosis.
Hypercalcemia of malignancy is a common paraneoplastic syndrome in patients with metastatic cancer and development of this complication is often associated with a poor prognosis.12 Parathyroid hormone-related peptide (PTHrP) plays a central role in humoral hypercalcemia of malignancy (HHM) and thorough evaluation to establish the cause of the hypercalcemia is essential, because some patients may have undiagnosed primary hyperparathyroidism.
Squamous cell cancers of the head and neck, esophagus, cervix and lung, renal cell carcinoma, breast and ovarian cancers account for the majority of solid malignancies causing PTHrP-mediated hypercalcemia.34 However, essentially any malignancy can cause this syndrome.
Accordingly, hypercalcemia is not an infrequent complication at the late stages of cancers of the digestive tract, with the exception of gastric cancer.5 Herein, we describe the rare paraneoplastic phenomenon of HHM in a patient with poorly differentiated gastric adenocarcinoma. This case is rare for two reasons: a) the histology was non-squamous and b) the location of the cancer (the stomach).
A 61-year-old African-American female with a past medical history of human immunodeficiency virus, hepatitis C, and hypertension, presented to the emergency room with complaints of shortness of breath of a few weeks' duration, which was progressively getting worse, along with fatigue, generalized weakness, intermittent epigastric discomfort, lightheadedness, and constipation. She denied any chest pain, palpitations, syncope, nausea, vomiting, fever, chills, night sweats, melena, bloody bowel movement, and changes in appetite or weight loss. On examination, pallor was noticed. Her bilateral lungs were resonant on percussion and clear on auscultation. Abdominal examination revealed mild epigastric tenderness on deep palpation; however, no guarding, rigidity, or rebound tenderness was noted. Bowel sounds were noted in all four quadrants and no hepatosplenomegaly was noticed. The evaluations of all other organ systems were unremarkable, including the results of the systemic lymph node examination and spinal tenderness for metastatic spread.
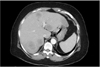
On initial laboratory evaluation, the following values were noted: hemoglobin, 8.4 g/dl (reference: 12~16 g/dl); hematocrit, 29% (reference: 36%~46%); aspartate transaminase, 74 U/L (reference: 13~39 U/L); calcium, 12.3 mg/dl (reference: 8.6~10.3 mg/dl); albumin, 2.0 mg/dl (reference: 3.5~5.0 mg/dl); corrected calcium, 13.9 mg/dl; corrected iron, 17 µg/dl; total iron-binding capacity, 518 µg/dl (reference: 250~400 µg/dl); iron saturation, 3% (reference: 15%~50%); ferritin, 192 ng/ml (reference: 14~233 ng/ml); vitamin B12, 490 pg/ml (reference: 211~911 pg/ml); folic acid, 14 ng/ml (reference: >4 ng/ml); carcinoembryonic antigen, 1.9 ng/ml (reference: 0~3 ng/ml); alpha-fetoprotein, 73 ng/ml (reference: 0.5~9.0 ng/ml), and lactate dehydrogenase, 750 µ/L (reference: 140~271 µ/L). Chest/abdominal/pelvic computed tomography (CT) showed extensive liver metastases, involving both lobes of the liver (Fig. 1). However, CT did not reveal any pulmonary disease or metastatic lesions.
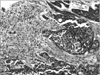
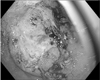
Consequently, the patient was admitted to our hospital. She was started on intravenous fluids, and received two units of packed red blood cells and one dose of pamidronate. Upon admission, colonoscopy and esophagogastroduodenoscopy (EGD) were performed. The colonoscopy findings were unremarkable while the EGD showed a large ulcerated mass at the greater curvature in the distal stomach (Fig. 2) and multiple biopsies were performed. In addition, she also underwent CT-guided liver biopsy. The pathology results of the liver biopsy showed poorly differentiated carcinoma (Fig. 3), while the gastric mass biopsy showed intestinal type, poorly differentiated, primary gastric adenocarcinoma (Fig. 4), which was confirmed by immunohistochemical analysis. Immunohistochemistry revealed positive staining for CDX2 (Ventana Medical Systems, Inc., Tucson, AZ, USA) and CK7 (Ventana Medical Systems, Inc.) (Fig. 5), while the expressions of HER2/neu (Ventana Medical Systems, Inc.) and neuroendocrine markers were negative. The tumor showed identical histologic features as the tumor identified from the liver biopsy specimen.
The calcium level slowly improved to 7.8 mg/dl, and the corrected calcium was 9.1 mg/dl on the 4th day of admission. Workup for primary hyperparathyroidism was negative, with the serum parathyroid hormone (PTH) level being low at 2.4 pg/ml (reference: 11.1~79.5 pg/ml) and the PTHrP level being elevated at 2.7 pmol/L (reference: <2 pmol/L).
Based on these findings, the patient was started on palliative chemotherapy with cisplatin and 5-fluorouracil. She received 4 cycles of chemotherapy with no response. During the treatment course, she was hospitalized a number of times due to severe hypercalcemia and altered mental status. Unfortunately, the patient died within 4 months of the diagnosis.
Malignancy-associated hypercalcemia is a common finding in patients with metastatic malignancy.3 The presence of hypercalcemia in a patient with malignancy signifies very poor prognosis, and almost 50% of these patients reportedly die within 30 days.2
Different mechanisms are responsible for the development of malignancy-associated hypercalcemia, including PTHrP-mediated humoral hypercalcemia, osteolytic bone metastases-related hypercalcemia, and 1,25-vitamin D-mediated hypercalcemia.
HHM is caused by systemic secretion of PTHrP by malignant tumor cells, which causes increased bone resorption and enhances renal retention of calcium.67 In HHM, hypersecretion of PTHrP is associated with physiologic suppression of PTH. One previous case report on hypercalcemia in a patient with metastatic gastric cancer due to hypersecretion of both serum PTH and PTHrP has been described.8 Both PTH and PTHrP interact with a common receptor, but their amino acid sequences and immunoreactivity differ, and there is no cross-reactivity in two-site assays for these two proteins. Elevated serum PTHrP has been reported in 50% to 90% of hypercalcemic patients with malignancy.9
The tumors that most commonly cause HHM are squamous-cell cancers (e.g., of the head and neck, esophagus, cervix, or lung), renal cell carcinoma, ovarian cancer, endometrial cancer, human T-lymphotropic virus-associated lymphoma, and breast cancer. Moreover, hypercalcemia is not an infrequent complication at the late stages of cancers of the digestive tract, with the exception of gastric cancer. Monno et al.5 performed a retrospective analysis on the incidence of hypercalcemia in 183 patients with malignancies of the gastrointestinal tract. The incidences of hypercalcemia by site were found to be 5/74 (6.8%), 1/16 (6.3%), 4/33 (12.1%), 3/15 (20.0%), 0/37 (0%), 0/2 (0%), 2/5 (40.0%), and 0/1 (0%) for liver, biliary tract, pancreatic, esophageal, stomach, duodenal, colon, and rectal carcinomas, respectively.
Elevated PTHrP without hypercalcemia has been described in association with gastric adenocarcinoma10 and one study showed that 71 patients with gastric adenocarcinoma expressed PTHrP in the tumor cells, without humoral hypercalcemia.11 Another study also found elevated serum PTHrP in approximately 17% of patients with gastro-esophageal cancers in the absence of hypercalcemia and reported that elevated serum PTHrP was associated with an adverse prognosis.12
In conclusion, abnormal PTHrP production can occur in malignant cells without causing hypercalcemia,13 and previous studies have shown that PTHrP expression is associated with poorly differentiated tumors. Especially, hypercalcemia is extremely rare in patients with metastatic gastric adenocarcinoma, despite expression or production of PTHrP by gastric malignant tumor cells.
Figures and Tables
References
1. Vassilopoulou-Sellin R, Newman BM, Taylor SH, Guinee VF. Incidence of hypercalcemia in patients with malignancy referred to a comprehensive cancer center. Cancer. 1993; 71:1309–1312.
2. Ralston SH, Gallacher SJ, Patel U, Campbell J, Boyle IT. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990; 112:499–504.
3. Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005; 352:373–379.
4. Burtis WJ, Brady TG, Orloff JJ, Ersbak JB, Warrell RP Jr, Olson BR, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990; 322:1106–1112.
5. Monno S, Nagata A, Furuta S. Hypercalcemia of cancer in the digestive tract. J Clin Gastroenterol. 1987; 9:78–82.
6. Nakayama K, Fukumoto S, Takeda S, Takeuchi Y, Ishikawa T, Miura M, et al. Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J Clin Endocrinol Metab. 1996; 81:607–611.
7. Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocaña A, Stewart AF. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab. 2003; 88:1603–1609.
8. Nakajima K, Tamai M, Okaniwa S, Nakamura Y, Kobayashi M, Niwa T, et al. Humoral hypercalcemia associated with gastric carcinoma secreting parathyroid hormone: a case report and review of the literature. Endocr J. 2013; 60:557–562.
9. Truong NU, deB Edwardes MD, Papavasiliou V, Goltzman D, Kremer R. Parathyroid hormone-related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003; 115:115–121.
10. Engelich G, Swan N, Hartshorn KL. Raised plasma parathyroid hormone related peptide in gastric adenocarcinoma. J Clin Pathol. 2000; 53:643–644.
11. Alipov GK, Ito M, Nakashima M, Ikeda Y, Nakayama T, Ohtsuru A, et al. Expression of parathyroid hormone-related peptide (PTHrP) in gastric tumours. J Pathol. 1997; 182:174–179.
12. Deans C, Wigmore S, Paterson-Brown S, Black J, Ross J, Fearon KC. Serum parathyroid hormone-related peptide is associated with systemic inflammation and adverse prognosis in gastroesophageal carcinoma. Cancer. 2005; 103:1810–1818.
13. Abdeen O, Pandol SJ, Burton DW, Deftos LJ. Parathyroid hormone-related protein expression in human gastric adenocarcinomas not associated with hypercalcemia. Am J Gastroenterol. 1995; 90:1864–1867.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download