Abstract
Primary squamous cell carcinoma (SCC) of the stomach is a very rare disease. However, the pathogenesis, clinical characteristics, and prognosis of gastric SCC are controversial and remain to be elucidated. Herein, we report a case of primary gastric SCC of the remnant stomach after subtotal gastrectomy. A 65-year-old man was admitted to our hospital due to epigastric discomfort and dizziness. He had undergone subtotal gastrectomy 40 years previously for gastric ulcer perforation. Endoscopy revealed a normal esophagus and a large mass in the remnant stomach. Abdominal computed tomography revealed enhanced wall thickening of the anastomotic site and suspected metachronous gastric cancer. Endoscopic biopsy revealed SCC. Total gastrectomy was performed with Roux-en-Y esophagojejunostomy. A 10-cm tumor was located at the remnant stomach just proximal to the previous area of anastomosis. Pathologic examination showed well-differentiated SCC extended into the subserosa without lymph node involvement (T3N0M0). The patient received adjuvant systemic chemotherapy with 6 cycles of 5-FU and cisplatin regimen, and he is still alive at the 54-month follow-up. According to the treatment principles of gastric cancer, early detection and radical surgical resection can improve the prognosis.
Primary squamous cell carcinoma (SCC) of the stomach is a rare type of gastric cancer. To date, only approximately 100 cases have been reported in the literature.1 Because most of the literature is limited to single case reports; the clinicopathologic characteristics, pathogenesis, best treatment option, and prognosis of patients with primary SCC of the stomach are controversial and remain to be elucidated. In addition, primary SCC of the remnant stomach after gastrectomy is an extremely rare occurrence.2345
Herein, we report a case of primary SCC of the remnant stomach after subtotal gastrectomy, which was treated with surgical resection and systemic adjuvant chemotherapy.
A 65-year-old man was admitted to our hospital owing to epigastric discomfort and dizziness. In addition, he experienced syncope a few days before admission and an 8 kg loss of body weight during the past 2 months. He had undergone subtotal gastrectomy 40 years previously for gastric ulcer perforation. He also had a history of anti-hypertensive medication use for the past 16 years. The patient had unremarkable family and social history and he appeared chronically ill. Upon physical examination, the vital signs were stable; however, he had pale conjunctiva. Any specific symptoms of gastrointestinal system involvement and signs of gross bleeding were not observed. Initial laboratory findings showed microcytic hypochromic anemia with very low levels of hemoglobin (5.9 g/dl) and serum iron (15 µg/dl). Other laboratory findings were within normal limits. The serum levels of the carcinoembryonic antigen and CA19-9 were 2.99 ng/ml and 6.4 kU/L, respectively.
A subsequent upper gastrointestinal endoscopy revealed a hemorrhagic and fungating tumor in the remnant stomach, near the previous gastrojejunostomy site (Fig. 1). Endoscopic biopsy of the tumor revealed a keratinizing SCC without Helicobacter pylori.
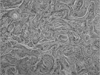
An abdominal computed tomography (CT) scan showed a heterogeneous enhanced wall thickening at the suspected gastroenterostomy site combined with several small lymph nodes along the left gastric vessels, retroperitoneal space, and gastrocolic trunk without any evidence of intra-abdominal distant metastasis (Fig. 2). Positron emission tomography (PET) CT of whole body was performed to exclude the possibility of another primary tumor or distant metastasis. A hypermetabolic mass was observed in the body of the stomach (maximum standard uptake value: 21.7) without distant metastasis (Fig. 3).
Complete total gastrectomy was performed with standard D2 dissection of the lymph nodes and Roux-en-Y esophagojejunostomy. Gross distant metastasis, peritoneal seeding, or direct invasion into the adjacent organs was not observed. When the resected specimen was opened, an approximately 10-cm ulcerofungating mass was observed just above the previous gastrojejunostomy site with a clear resection margin (Fig. 4).
Pathologic examination of the resected specimen revealed a keratinized well-differentiated SCC with keratin pearl in the remnant stomach (Fig. 5). The tumor extended into the subserosal layer without lymphovascular invasion or metastasis to th5e9 regional lymph nodes (T3N0M0, TNM stage IIA). Immunohistochemistry staining showed that the tumor cells were positive for P63, K903, and cytokeratin (CK) 5/6; and negative for CK 7 and CK 20. The patient was discharged without complications 2 weeks after the surgery.
The patient received systemic adjuvant chemotherapy with 6 cycles of 5-FU and cisplatin regimen (1,000 mg/m2 of 5-FU on day 1~3, 800 mg/m2 of cisplatin on day 2, every 4 weeks) and is still alive and recurrence-free at the 54-month follow-up.
Primary SCC of the stomach is a very rare malignant tumor that accounts for 0.04% to 0.07% of all gastric carcinomas.1678 Since the first case of primary gastric SCC was described in 1905, less than 100 cases have been reported in the literature to date.1 In addition, SCC of the remnant stomach following gastrectomy is extremely rare, and has been described in less than 5 reported cases.2345 Because most reports on the primary gastric SCCs are restricted to single case reports, the clinical features, pathologic characteristics, pathogenesis, optimal treatment method, and prognosis associated with the disease are controversial and remain to be elucidated.
Parks9 proposed that primary gastric SCC should satisfy the following diagnostic criteria: (1) the tumor must not be located in the cardia; (2) the tumor must not extend into the esophagus; and (3) there should be no evidence of SCC in any part of the body.10 Other diagnostic criteria were proposed by the Japanese Gastric Cancer Association, which included the following: (1) all tumor cells are SCC cells, with no adenocarcinomatous components in any sections and (2) distinct evidence that SCC arises directly from the gastric mucosa.11 The pathologic cell type, tumor location, and PET-CT findings of our patient were consistent with both diagnostic criteria.
Although the pathogenesis of primary gastric SCCs is still unknown, several hypotheses concerning the origin of the squamous cancer cell have been proposed, and are as follows: (1) squamous differentiation in the preexisting adenocarcinoma; (2) squamous metaplasia of the gastric mucosa before malignant transformation; (3) multipotent stem cells capable of differentiation into any cell type; (4) nest of ectopic squamous epithelium in gastric mucosa; (5) gastric vascular endothelial cell; and (6) epstein-barr virus or human papilloma virus in some patients.157812131415 It has been reported that squamous metaplasia of the normal gastric mucosa could arise in special circumstances (gastric ulcer, corrosive burn, congenital syphilis, chemoradiotherapy, and long-term treatment with cyclophosphamide).1617 For our patient, we carefully assumed that squamous metaplasia of the remnant gastric mucosa might have occurred owing to the continuous irritation from the bile as a result of gastrojejunostomy, from which cancer cells could have developed subsequently.
Wakabayashi et al.8 reviewed 56 cases of primary gastric SCCs in Japan. He reported that the mean age at onset was 64.7 years (range, 29~81 years) and the male-to-female ratio was 44:12. The tumor was most commonly located at the upper third of the stomach (57.1%). The mean tumor size was 6.6 cm and the type 2 was most frequent macroscopic type. More than half of the cases showed extension beyond T4 invasion (T4a, 14% and T4b, 45%), which resulted in poor surgical curability. Gao et al.1 reported, through the review of the most recent 11 cases, that abdominal pain was the most frequently observed clinical presentation, the mean age was 63.8 years, and the mean tumor size was 8.2 cm. Hwang et al.7 also reported similar results in their review of 90 cases. Recent studies suggested that the clinical characteristics of the primary gastric SCCs are more evident in the 6th to 7th decades of life, show male predominance, are commonly located in the upper stomach, and have large tumor size and advanced stage at the time of diagnosis. Nevertheless, a case of a primary gastric SCC in a 17-year-old boy has been reported.18
Although the clinical manifestations of primary gastric SCCs are similar to those of adenocarcinoma of the stomach, it might have unusual features. Our patient was admitted to the hospital owing to symptoms of chronic hemorrhage of the tumor. Other researchers have also reported hemorrhage to be the chief clinical presentation in patients with primary gastric SCC.1619 Primary gastric SCC might resemble a submucosal mass on endoscopy; therefore, it could be misdiagnosed as gastrointestinal stromal tumor or malignant lymphoma of the stomach.72021 Huge exophytic growth, paraneoplastic leukocytosis, and hypercalcemia in primary gastric SCCs also have been reported as rare clinical manifestations.10222324
Histopathological criteria for the diagnosis of a primary gastric SCC was defined by Boswell and Helwig6 in 1965. At least one of these criteria should be satisfied for the confirmation of diagnosis, which include keratinizing cell masses with keratin pearl, a mosaic cell arrangement, intercellular bridges, and high concentrations of sulfhydryl or disulphide bonds. Keratin pearl arranged in a mosaic pattern in the squamous cell masses confirmed the diagnosis of primary SCC in the patient. In addition, immunohistochemical staining of the squamous tumor cells revealed that they were positive for P63, K903, and CK 5/6, which are indicative of SCC and are negative for CK 7 and CK 20 (for adenocarcinoma). These results strongly supported the diagnosis.
The standard treatment for primary gastric SCC is radical gastrectomy with adequate lymph node dissection. However, prognosis seems unfavorable compared to adenocarcinoma of the stomach, because primary gastric SCCs are usually in the advanced stage at the time of diagnosis.178 Surgery alone might be insufficient for achieving disease-free survival; therefore, various adjuvant therapy regimens were administered for supportive treatment. Most results were not promising and an ideal chemotherapy regimen for the treatment of gastric SCC has not been identified.
Marubashi et al.25 and Modi et al.26 reported the effectiveness of neoadjuvant chemotherapy for gastric SCC, and Yanagisawa et al.27 reported a complete response of primary gastric SCC to chemotherapy with docetaxel and cisplatin plus 5-fluorouracil. The patient is still alive after receiving adjuvant chemotherapy with FP and he was recurrence-free at the 54-month follow-up. Considering the favorable response of SCC to radiotherapy, chemoradiotherapy could be one of the treatment options.
In conclusion, primary SCC of the stomach is rare; however, it might be aggressive, and its pathogenesis and the optimal treatment option remain to be elucidated. According to the treatment principles of gastric cancer, early detection and radical surgical resection could improve the prognosis.
Figures and Tables
Fig. 1
Endoscopy shows a hemorrhagic and fungating tumor in the remnant stomach near the site of a previous gastrojejunostomy.
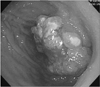
Fig. 2
Abdominal computed tomography scan shows heterogeneous wall thickening of the remnant stomach.

Fig. 3
A hypermetabolic mass of the stomach (maximum standard uptake value: 21.7) was identified via whole body positron emission tomography. Another primary cancer or distant metastases were excluded.

References
1. Gao S, Chen D, Huang L, Dai R, Shan Y. Primary squamous cell carcinoma of the stomach: a case report and literature review. Int J Clin Exp Pathol. 2015; 8:9667–9671.
2. Ruck P, Wehrmann M, Campbell M, Horny HP, Breucha G, Kaiserling E. Squamous cell carcinoma of the gastric stump. A case report and review of the literature. Am J Surg Pathol. 1989; 13:317–324.
3. Wegstapel H, Thomas WE. Squamous cell carcinoma of the stomach following gastric surgery. Br J Clin Pract. 1990; 44:736–737.
4. Piper MH, Ross JM, Bever FN, Shartsis JM, Mohammadi D. Primary squamous cell carcinoma of a gastric remnant. Am J Gastroenterol. 1991; 86:1080–1082.
5. Tokuhara K, Nakano T, Inoue K, Nakane Y, Kwon AH. Primary squamous cell carcinoma in the gastric remnant. Surg Today. 2012; 42:666–669.
6. Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965; 18:181–192.
7. Hwang SH, Lee JH, Kim K, Shin DH, Kim JY, Sol MY, et al. Primary squamous cell carcinoma of the stomach: a case report. Oncol Lett. 2014; 8:2122–2124.
8. Wakabayashi H, Matsutani T, Fujita I, Kanazawa Y, Nomura T, Hagiwara N, et al. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014; 14:58–62.
9. Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol Radium Ther Nucl Med. 1967; 101:447–449.
10. Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis: a case report and review of literature. Hum Pathol. 2009; 40:1494–1498.
11. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 13th ed. Tokyo: Kanchara;1999.
12. Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969; 24:985–995.
13. Muto M, Hasebe T, Muro K, Boku N, Ohtsu A, Fujii T, et al. Primary squamous cell carcinoma of the stomach: a case report with a review of Japanese and Western literature. Hepatogastroenterology. 1999; 46:3015–3018.
14. Takita J, Kato H, Miyazaki T, Nakajima M, Fukai Y, Masuda N, et al. Primary squamous cell carcinoma of the stomach: a case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology. 2005; 52:969–974.
15. Amuluru K, Gupta H. Primary squamous cell carcinoma of the stomach: a case report. J Gastrointest Cancer. 2010; 41:24–26.
16. Dursun M, Yaldiz M, Işikdoğan A, Yilmaz G, Canoruç F, Ormeci N, et al. Primary squamous cell carcinoma of the stomach: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003; 15:329–330.
17. Sakemi R, So S, Morimitsu Y, Imada H, Ishihara H, Kuhara K, et al. Endoscopic submucosal dissection of squamous cell carcinoma in the upper stomach 5 years after chemoradiotherapy for adenocarcinoma. Clin J Gastroenterol. 2014; 7:310–315.
18. Schwab G, Wetscher G, Dietze O, Schmid K, Pointner R. Primary squamous cell carcinoma of the stomach in a seventeen-year-old boy. Surg Today. 1992; 22:561–564.
19. Karaca G, Pekcici MR, Özer H, Köklü S, Kavlakoğlu B, Astarci M, et al. Primary squamous cell carcinoma of the stomach in a 68-years-old man. Geriatr Gerontol Int. 2011; 11:119–120.
20. Jeong GA, Min YD, Lim SC. Primary squamous cell carcinoma of the stomach that was misdiagnosed as a submucosal tumor. J Korean Surg Soc. 2007; 73:439–442.
21. von Waagner W, Wang Z, Picon AI. A rare case of a primary squamous cell carcinoma of the stomach presenting as a submucosal mass. Case Rep Surg. 2015; 2015:482342.
22. Hara J, Masuda H, Ishii Y, Aoki N, Nakayama H, Komura K, et al. Exophytic primary squamous cell carcinoma of the stomach. J Gastroenterol. 2004; 39:299–300.
23. Lee JH, Yang KY, Kim BH, Koh SJ, Kang HY, Keam B, et al. A case of exophytic squamous cell carcinoma of the stomach. Korean J Gastrointest Endosc. 2006; 33:357–360.
24. Wu XD, Zhou Y, Fan RG, Zhou B, Shi Q, Jia J. Primary squamous cell carcinoma of the stomach presenting as a huge retroperitoneal tumor: a case report. Rev Esp Enferm Dig. 2016; 108:283–284.
25. Marubashi S, Yano H, Monden T, Tateishi H, Kanoh T, Iwazawa T, et al. Primary squamous cell carcinoma of the stomach. Gastric Cancer. 1999; 2:136–141.
26. Modi Y, Shaaban H, Parikh N, Guron G, Maroules M. Primary pure squamous cell carcinoma of the stomach treated with neoadjuvant chemotherapy and surgical resection. Indian J Cancer. 2015; 52:145.
27. Yanagisawa S, Tsuchiya S, Kaiho T, Togawa A, Shinmura K, Okamoto R, et al. Histological complete response in a case of primary squamous cell carcinoma of the stomach treated by chemotherapy with docetaxel and cisplatin plus 5-fluorouracil. Gan To Kagaku Ryoho. 2010; 37:307–310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download