Abstract
We report the case of a patient with gastric adenocarcinoma with multiple liver metastases. This patient showed complete remission for more than 68 months after S-1/cisplatin combination chemotherapy and radical total gastrectomy. The patient, a 63-year-old man, presented with dyspepsia and difficulty in swallowing. Endoscopic findings showed a huge ulcero-infiltrative mass at the lesser curvature of the mid-body, extending to the distal esophagus. Biopsy revealed a poorly differentiated tubular adenocarcinoma. An abdominal computed tomography scan demonstrated multiple hepatic metastases. S-1/cisplatin combination chemotherapy was initiated, and following completion of six cycles of chemotherapy, the gastric masses and hepatic metastatic lesions had disappeared on abdominal computed tomography. Radical total gastrectomy and D2 lymphadenectomy combined with splenectomy were performed. The patient underwent three cycles of S-1/cisplatin combination chemotherapy followed by tegafur-uracil therapy for 1 year. He remained in complete remission for more than 68 months after surgery.
Gastric cancer is the fifth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide.1 Surgery is the main treatment strategy for gastric cancer; only R0 resection anticipates a potential cure. It is very difficult to achieve complete remission (CR) in metastatic gastric cancer; palliative chemotherapy is the main treatment option. A median overall survival of 8 to 13 months has been reported in patients undergoing chemotherapy, while this drops to 3 to 5 months for those treated with best supportive care.234 Rare reports have presented good therapeutic results for metastatic gastric cancer treated with chemotherapy and/or surgical treatment.567 However, in most of these reports, CR did not last for more than 5 years.
Here, we report a case of gastric adenocarcinoma with multiple liver metastases that showed CR for more than 68 months after S-1/cisplatin combination chemotherapy followed by radical total gastrectomy.
A 63-year-old man presented with anorexia, dyspepsia, and difficulty swallowing in January 2010. His performance status was 2 according to the criteria of the Eastern Cooperative Oncology Group. Blood count analysis results were as follows: hemoglobin 9.6 g/dl, mean corpuscular volume 72.7 fL, leukocyte count 6,000/mm3, and platelet count 253,000/mm3. Liver function test results were as follows: total protein 7.0 g/dl, albumin 4.1 g/dl, total bilirubin 1.1 g/dl, aspartate aminotransferase 36 IU/L, alanine aminotransferase 27 IU/L, alkaline phosphatase 86 IU/L, carcinoembryonic antigen 1.58 ng/dl, and cancer antigen 19-94.6 IU/ml.
Endoscopic findings showed a huge ulcero-infiltrative mass at the lesser curvature of the mid-body, extending to the distal esophagus (Fig. 1A, B). Biopsy revealed a poorly differentiated tubular adenocarcinoma (Fig. 2).
An abdominal computed tomography (CT) scan demonstrated irregular wall thickening on the lesser curvature side of the gastric upper body with perigastric fat invasion and multiple metastases to neighboring lymph nodes (Fig. 3A). The CT scan also revealed multiple hepatic metastatic lesions (1.0 to 1.6 cm in size over the entire liver) (Fig. 3A~C). A diagnosis of stage IV (cT3N3M1) advanced gastric cancer was made according to the 7th American Joint Committee on Cancer (AJCC) system.
We administered S-1/cisplatin combination chemotherapy. For each cycle, oral S-1 (80 mg/m2) was administered for 2 weeks, followed by a 1-week drug holiday. Intravenous cisplatin (60 mg/m2) was administered on day 1 of each cycle after adequate premedication and hydration.
In March 2010, after completion of three cycles of chemotherapy, an abdominal CT scan showed improvement in the gastric cardia and body mass. Additionally, the hepatic metastatic lesions had disappeared, with the exception of the lesions in liver segments 3 and 6, which measured less than 1 cm in size (Fig. 3D~F). After an additional three cycles of chemotherapy, another abdominal CT scan was performed in May 2010. This showed that the gastric mass had almost disappeared and that the hepatic metastatic nodules that had been previously observed were no longer present (Fig. 4A, B). To confirm the absence of the hepatic metastatic lesions, we performed a magnetic resonance imaging scan of this liver; this did not detect any metastatic lesions in the liver. Follow-up endoscopy showed that the gastric cardia mass had disappeared and an erosive lesion at the site of the previous mass was all that remained (Fig. 1C, D). The patient was transferred to the surgery department.
Radical total gastrectomy with Roux-en-Y esophagojejunostomy and D2 lymphadenectomy combined with splenectomy was performed in May 2010. Pathologic findings showed primary tumor glands extending to the submucosa, and metastatic disease was observed in three out of 33 resected lymph nodes. The pathologic stage was ypT1bN2M0, stage IIA, according to the 7th AJCC system
We planned to administer S-1/cisplatin combination chemotherapy again. However, after three cycles of chemotherapy, the patient developed grade 3 peripheral neuropathy in both hands and refused chemotherapy. We recommended continuous tegafururacil (UFT) monotherapy (360 mg/m2/day daily) for 1 year.
Every 3 months, an abdominal CT or positron emission tomography (PET)-CT scan was performed. There was no evidence of recurrent tumor until January 2016. The patient had maintained CR for more than 68 months after surgery (Fig. 4C, D).
Neoadjuvant chemotherapy is recommended for the treatment of advanced gastric cancer in order to improve resectability and clinical outcome. Some reports have shown favorable results with S-1/cisplatin combination chemotherapy in the neoadjuvant setting. Kochi et al.8 retrospectively compared a neoadjuvant S-1/cisplatin combination chemotherapy group and a surgery-only group. The response rate of patients in the neoadjuvant chemotherapy group was 78.6%, and they demonstrated a significantly better survival rate than the patients in the surgery-only group (P=0.03). Satoh et al.9 retrospectively reviewed the cases of 45 consecutive patients with advanced gastric cancer who were treated with S-1/cisplatin neoadjuvant chemotherapy, and reported that the response rate and downstaging rates in these patients were 43.5% and 55.6%, respectively.
Similar to our case, other studies have reported a dramatically favorable result with S-1 monotherapy. Iwahashi et al.5 reported the case of a patient with advanced gastric cancer with peritoneal dissemination treated with S-1 and low dose cisplatin who achieved CR. In that case, surgery was performed after two cycles of chemotherapy, and no tumor cells were detected in the gastric primary lesion, metastatic lymph nodes, or disseminated peritoneal tumors on histological examination, suggesting pathological CR.
Mitomi et al.6 reported a CR duration of 50 months in a patient with advanced gastric cancer with paraaortic lymph node metastases after S-1 monotherapy followed by total gastrectomy. Suzuki et al.7 reported that a patient treated with S-1 monotherapy maintained CR for 4 years without gastrectomy. As observed in these cases, S-1 may induce a very surprising response in a subgroup of advanced gastric cancer patients. This requires further study.
Currently, the most frequently used adjuvant chemotherapy regimens for gastric cancer after curative surgery are S-1 monotherapy and capecitabine/oxaliplatin combination chemotherapy. Patients with stage II or III gastric cancer who underwent gastrectomy with extended (D2) lymph node dissection followed by adjuvant S-1 chemotherapy showed a 3-year overall survival rate of 80.1%.10
Our patient refused to take the oral S-1 drug. Furthermore, capecitabine/oxaliplatin combination therapy could not be administered due to the development of grade 3 peripheral neuropathy. It was previously demonstrated in a phase 3 factorial randomized controlled trial that UFT is not inferior to S-1 (SAMIT: sequential paclitaxel followed by UFT or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer); the 3-year disease-free survival rate for the UFT group was 53.0% (95% confidence interval [CI] 49.2~56.6), while it was 58.2% for the S-1 group (54.4~61.8; hazard ratio 0.81, 95% CI 0.70~0.93, P=0.0048; P=0.151).11 Hence, we administered adjuvant UFT therapy instead of adjuvant S-1 monotherapy.
To our knowledge, this is the first report of a patient with advanced gastric cancer with hepatic metastasis who was treated with S-1/cisplatin chemotherapy followed by total gastrectomy and maintained CR for more than 68 months. One study reported a case with hepatic metastatic lesions that showed metabolic remission on 18F-fluorodeoxyglucose PET after treatment with combination chemotherapeutic agents including oxaliplatin, 5-fluorouracil, and leucovorin (modified FOLFOX-6 regimen); however, the patient did not achieve CR and the remission duration was 12 months.12 In our case, a liver biopsy was not performed. However, when the patient was diagnosed with liver metastasis based on the CT images, there was no evidence of infection (body temperature 36.7℃, C-reactive protein 0.08 mg/dl) such as liver abscess. Additionally, the eosinophil count was within the normal range (eosinophils 4.9%, total eosinophil count 290/mm3); hence, we could rule out eosinophilic liver infiltration. Furthermore, after completion of three cycles of chemotherapy, another abdominal CT scan was performed. CT images revealed that some of the hepatic metastatic lesions had disappeared; those in liver segments 3 and 6 lesions persisted, although they measured less than 1 cm in size. As a result, we could only consider liver metastasis for the pathology of the liver lesions at the time of the initial diagnosis, and these lesions were expected to respond to S-1/cisplatin chemotherapy.
In conclusion, we report the case of a patient with stage IV gastric cancer with liver metastases who was treated with S-1/cisplatin combination chemotherapy and radical total gastrectomy, achieved CR, and maintained remission for more than 68 months after surgery.
Figures and Tables
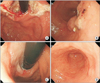 | Fig. 1Initial (A, B) and follow-up (C, D) endoscopic findings after six cycles of S-1/cisplatin combination chemotherapy: (A) An ulcero-infiltrative mass at the lesser curvature of the mid-body. (B) An ulcero-fungating mass extending to the distal esophagus. (C) Disappearance of the gastric cardia mass, and erosive lesions at the site of the previous mass. (D) Ulcer scar without a definite mass-like lesion. |
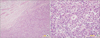 | Fig. 2Microscopic findings at the time of diagnosis. Poorly differentiated tubular adenocarcinoma (A: H&E, ×100; B: H&E, ×400). |
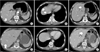 | Fig. 3Initial abdominal computed tomography scan (A~C) and follow-up scan after three cycles of S-1/cisplatin combination chemotherapy (D~F): (A) Irregular wall thickening on the lesser curvature side of the gastric upper body with perigastric fat invasion, multiple metastases to neighboring lymph nodes, and metastatic lesion in liver segment 6. (B) Multiple hepatic metastatic lesions and small low attenuating nodules with rim enhancement in liver segment 3. (C) Hypodense hepatic metastatic lesion in liver segment 3. (D) Scan shows that the metastatic lesion in liver segment 6 decreased in size from 1.2 cm to less than 1.0 cm. (E) Disappearance of hepatic metastatic nodules that had been previously observed. (F) Scan shows that the metastatic lesion in liver segment 3 decreased in size from about 1.1 cm to less than 1.0 cm. |
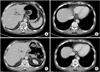 | Fig. 4Follow-up abdominal computed tomography scans after six cycles of S-1/cisplatin combination chemotherapy (A, B) and 68 months after surgery (C, D): (A) Scan shows that the gastric mass and neighboring lymph nodes have almost disappeared. (B) Scan shows that the hepatic metastatic nodules that had been previously observed have disappeared (C). Radical total gastrectomy with Rouxen-Y esophagojejunostomy combined with splenectomy was performed. Scan shows that there was no evidence of tumor recurrence 68 months after surgery. (D) There was no recurrence of the hepatic metastatic lesions and the patient maintained complete remission for 68 months. |
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
2. Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168.
3. Rosati G, Ferrara D, Manzione L. New perspectives in the treatment of advanced or metastatic gastric cancer. World J Gastroenterol. 2009; 15:2689–2692.
4. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221.
5. Iwahashi M, Nakamori M, Tani M, Yamaue H, Sakaguchi S, Nakamura M, et al. Complete response of highly advanced gastric cancer with peritoneal dissemination after new combined chemotherapy of S-1 and low-dose cisplatin: report of a case. Oncology. 2001; 61:16–22.
6. Mitomi H, Kishimoto I, Amemiya A, Kaneda G, Adachi K, Shimoda T, et al. Advanced gastric cancer showing long-term complete remission in response to S-1 monotherapy: two case reports. Cases J. 2008; 1:405.
7. Suzuki Y, Kawasaki N, Ishibashi Y, Yanaga K, Kashiwagi H, Koba K, et al. A case of stage IV gastric cancer: long term remmision achieved with S-1 mono chemotherapy. JMAJ. 2006; 49:219–223.
8. Kochi M, Fujii M, Kanamori N, Kaiga T, Takahashi T, Kobayashi M, et al. Neoadjuvant chemotherapy with S-1 and CDDP in advanced gastric cancer. J Cancer Res Clin Oncol. 2006; 132:781–785.
9. Satoh S, Hasegawa S, Ozaki N, Okabe H, Watanabe G, Nagayama S, et al. Retrospective analysis of 45 consecutive patients with advanced gastric cancer treated with neoadjuvant chemotherapy using an S-1/CDDP combination. Gastric Cancer. 2006; 9:129–135.
10. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.
11. Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014; 15:886–893.
12. Yoon SY, Kim SY, Cho YH, Chung HW, So Y, Lee HM. Hepatic metastases of gastric adenocarcinoma showing metabolic remission on FDG-PET despite an increase in size on CT. Cancer Res Treat. 2009; 41:100–103.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download