Abstract
Epstein-Barr virus-associated gastric carcinoma (EBVaGC) is one of the four subtypes of gastric carcinoma (GC), as defined by the novel classification recently proposed by The Cancer Genome Atlas. EBVaGC has several clinicopathological features such as longer survival and higher frequency of lymphoepithelioma-like carcinoma (LELC) and carcinoma with Crohn's disease-like lymphoid reaction that distinguish it from EBV-negative GC. The intensity and pattern of host cellular immune response in GC have been found to significantly correlate with the prognosis of patients with GC, suggesting that immune reaction and tumor microenvironment have critical roles in the progression of GC, and in particular, EBVaGC. Here, we reviewed the cellular and molecular mechanisms underlying prominent immune reactions in patients with EBVaGC. In EBVaGC, deregulation of the expression of immune response-related genes promotes marked intra- or peritumoral immune cell infiltration. The expression of programmed death receptor-ligand 1 is known to be increased in EBVaGC, and therefore, it has been proposed as a favorable prognostic factor for patients with EBVaGC, albeit some data supporting this claim are controversial. Overall, the underlying mechanisms and clinical significance of the host cellular immune response in patients with EBVaGC have not been thoroughly elucidated. Therefore, further research is necessary to better understand the role of tumor microenvironment in EBVaGC.
Infection with the Epstein-Barr virus (EBV), a ubiquitous oncogenic g-type herpes virus, is implicated in the etiology of several lymphoid and epithelial malignancies, including Burkitt's lymphoma, Hodgkin's lymphoma, nasal NK/T cell lymphoma, nasopharyngeal carcinoma, and a subset of gastric carcinomas (GCs).123 EBV-associated GC (EBVaGC) is defined as the monoclonal proliferation of carcinoma cells with latent EBV infection, which can be demonstrated by in situ hybridization targeted at EBV-encoded small RNA (EBER). In EBVaGC, virtually all cancer cells contain EBV DNA sequences, and the EBV terminal repeat sequences have uniform lengths, implying that the tumor can arise from a single EBV-infected cell and that the EBV genome persists during malignant transformation and proliferation.45 About 10% (1.3% to 20.1%) of GCs are EBV-positive;678 however, the prognostic effect of EBV positivity in GC is controversial. While some studies have shown significantly better prognosis in EBVaGC than in EBV-negative GC (EBVnGC),9101112 other studies failed to observe this prognostic difference.13141516 Nonetheless, a recent international large-scale meta-analysis of 4,599 cases revealed that patients with EBVaGC had longer survival than those with EBVnGC.17
Recently, The Cancer Genome Atlas (TCGA) research network proposed a new molecular classification, according to which GC is divided into four subtypes: EBV-positive tumors, microsatellite-instable tumors, genomically stable tumors, and tumors with chromosomal instability. EBV-positive tumors display recurrent PIK3CA mutations, extreme DNA hypermethylation, and enhanced expression of JAK2, CD274 (also known as programmed death receptor-ligand [PD-L] 1), and PDCD1LG2 (also known as PD-L2).18
Histologically, EBVaGC is characterized by marked intra- or peritumoral immune cell infiltration. In our previous study, we classified EBVaGC into three histological subtypes according to the cellular immune responses that affect prognosis: lymphoepithelioma-like carcinoma (LELC), carcinoma with Crohn's disease-like lymphoid reaction (CLR), and conventional adenocarcinoma (CA).19 The prognostic value of immune reactions in EBVaGC suggests that tumor microenvironment is a very important factor in the progression of EBVaGC.
Numerous studies have focused on the relationship between host cellular immune responses and EBVaGC prognosis. In this article, we reviewed the clinicopathological features of EBVaGC and EBV-associated immune responses in patients with EBVaGC.
According to the meta-analysis of 15,952 cases, EBV-positive GC occurs more frequently in male than in female patients. EBV-positive tumors usually arise in the cardia or the body of the stomach rather than in the antrum. Tumors in the post-surgical gastric stump/remnants are four times more likely to be EBV-positive than other GCs.20 There was no significant correlation between age and EBV positivity.16192021 Several studies have found an apparently higher incidence of synchronous multiple carcinomas in EBVaGC than in EBVnGC.222324 Smoking is one of the risk factors of EBVaGC.25 The influence of Helicobacter pylori infection on EBVaGC emergence is controversial.721262728 The presence of EBV-positive tumors negatively correlates with the TNM stage parameter as well as with the values of its individual components (primary tumor site, regional lymph node involvement, or presence of distant metastasis).17 Overall, EBV positivity is associated with favorable prognosis.9101112
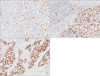
In histological examinations, marked intra- or peritumoral immune cell infiltration is usually detected in EBVaGC samples. Previously, we divided EBVaGC into three histological subtypes according to the microscopic characterization of host cellular immune responses: LELC, CLR, and CA. Typical LELC was defined by (1) a well-defined tumor margin, (2) dense lymphocytic infiltration when the number of tumor-infiltrating lymphocytes (TIL) was greater than that of tumor cells, (3) indistinct cytoplasmic borders and a syncytial growth pattern with poorly formed glandular structures, and (4) absence of desmoplasia (Fig. 1A). CLR was characterized by (1) patchy lymphocytic infiltration with three or more lymphoid follicles with active germinal centers per tissue section at the advancing edge of the tumor, (2) lower number of lymphocytes compared to tumor cells, (3) frequent tubule or gland formation, (4) the presence or complete absence of minimal desmoplasia, and (5) increased intratumoral lymphocyte infiltration (Fig. 1B). Finally, cases showing infiltration of scattered lymphocytes with prominent desmoplasia in the absence of lymphoid follicles, or with only one or two lymphoid aggregates per tissue section, were classified as CA (Fig. 1C).19 The prognosis was affected by the intensity and pattern of the inflammatory response. Among them, LELC cases had the best prognosis, followed by patients with CLR, who, in turn, had better survival rates than those with CA.17192930
Tumor microenvironment can play a critical role in patient outcome. TILs constitute the principal cellular component of the immune-active tumor microenvironment and their presence contributes to improved survival of patients with cancer. The number of TILs increases in EBVaGC, especially in LELC or CLR. Several studies found that EBVaGC is often accompanied by more extensive infiltration of CD8-positive cytotoxic T cells and a higher number of mature dendritic cells than EBVnGC.141530313233
Infiltrating immune cells at least partially contribute to antitumor immunity by promoting the eradication of EBV-positive malignant cells.193134 However, neither the mechanisms by which carcinoma cells are killed nor the biochemical cascades that allow them to evade the host immune response during tumor development and progression have been properly determined.
In 1998, transplantable human EBVaGC was designed and propagated in mice with severe combined immunodeficiency.35 The only cytokine gene that showed markedly higher expression in EBVaGC tumor strains compared to EBVnGC strains was IL1B that encodes interleukin-1β (IL-1β).36 IL-1β recruits numerous nonspecific lymphocytes to prevent direct contact between EBV-specific cytotoxic T cells and tumor cells.36
Using a cohort of GC RNA-Seq data sets from TCGA, Strong et al.37 performed a quantitative assessment of EBV gene expression in GC and analyzed EBV-associated alterations of cellular pathways. They found that EBV infection is associated with lower expression of tumor regulatory genes. Furthermore, samples with high levels of EBV gene expression displayed more extensive immune cell infiltration and higher interferon-gamma expression than samples with low or no EBV gene expression. These findings may explain the favorable prognosis of EBVaGC. On the other hand, high interferon-gamma levels induced the expression of indoleamine 2,3-dioxygenase (IDO1), a potent immune cell inhibitor, in EBVaGC cells. This circumstance may explain relative resistance of EBVaGC tumors to elevated numbers of immune cells.37
Kim et al.38 generated a gene expression profile analysis to compare tumor and non-tumor gastric tissues from 12 patients with EBVaGC and 14 with EBVnGC. It was revealed that EBVaGC cases had a higher degree of genetic homogeneity than EBVnGC cases. Deregulation of gene expression affected fewer genes in patients with EBVaGC than in those with EBVnGC. Furthermore, the majority of significant altered expression signals in EBVaGC are related to the immune response genes, especially the genes encoding proteins from cytokine (chemokine) pathways. These changes could recruit reactive immune cells to the intra- or peritumoral area of EBVaGC and this circumstance might contribute to longer survival of patients with EBVaGC compared to that of patients with EBVnGC.38
At the genomic level, EBVaGC is accompanied by a relatively frequent recurrent amplification of the 9p24.1 locus containing the CD274 gene encoding PD-L1, which is also known as B7-H1.18 PD-L1 expression on tumor cells is known to play an important role in immune evasion. This is accomplished through the interaction with the co-inhibitory molecule programmed death receptor-1 (PD-1) expressed by T cells.39 The PD-1/PD-L1 interaction inhibits T lymphocyte proliferation, survival, and effector functions such as cytotoxicity and cytokine release. In addition, it induces apoptosis of tumor-specific T cells, promotes the differentiation of CD4+ T cells into Foxp3+ regulatory T (Treg) cells, and increases the resistance of tumor cells to cytotoxic T lymphocyte attacks.40 This interaction mediates the suppression of T cell functions, and thus reduces T-cell receptor (TCR)-mediated proliferation and cytokine production.39 Actually, we observed markedly increased levels of CD4- and CD8-positive T cells and a moderate increase of CD20 positive B cells around EBV-positive tumor cells. However, we observed very small numbers of CD1a positive dendritic cells (Fig. 2).
Blockade of PD-L1/PD-1 interaction may improve the efficacy of adoptive cell therapies in certain malignancies. Additionally, a correlation between prognosis and the level of PD-L1 expression has been reported in several human malignancies.414243444546474849505152535455 Although some controversy remains, many studies have shown that PD-L1 overexpression is associated with worse prognosis in many types of cancer.56 Two published studies of GC used immunohistochemical analysis and showed that abnormally high levels of PD-L1 are associated with poor prognosis,4142 while a recent study with 243 patients with curatively resected GC showed better disease-free survival and overall survival in patients with PD-L1 expression.57 In TCGA data, PD-L1/2 expression was elevated in EBVaGCs, in which IL-12-mediated signaling signatures induced robust immune cell presence.18 When coupled with the evidence of PD-L1/2 overexpression, this finding adds rationale for testing immune checkpoint inhibitors in EBVaGC.18
The findings described above do not offer complete explanation for all specific clinicopathological features of EBVaGC. Future studies are necessary to clarify the precise mechanisms of the immune reaction to EBV infection, additional clinical aspects of EBVaGC, and the therapeutic strategy for patients with EBVaGC.
One of the most distinctive clinicopathological features of EBVaGC is a prominent cellular immune reaction, reflected in the higher frequency of LELC and CLR compared to the occurrence of these signs in EBVnGC. This histological hallmark supports the hypothesis that interactions between tumor cells and tumor microenvironment play a critical role in the progression of EBVaGC. The intensity and pattern of the inflammatory response are strongly associated with the prognosis of GC. Accordingly, establishing the identity of cellular immune pathways affecting EBV infection during GC will be helpful for selecting appropriate targets for therapeutic intervention against EBVaGC. EBVaGC can be detected by a relatively simple method using EBER in situ hybridization; thus, any advances in the immunotherapy of EBVaGC can be readily extended to many patients with GC worldwide. Further studies are necessary to clarify the mechanisms underlying the cellular immune response in EBVaGC.
Figures and Tables
References
1. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt's lymphoma. Lancet. 1964; 1:702–703.
2. Epstein MA, Barr YM. Cultivation in vitro of human lymphoblasts from burkitt's malignant lymphoma. Lancet. 1964; 1:252–253.
3. Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004; 4:757–768.
4. Fukayama M, Hayashi Y, Iwasaki Y, Chong J, Ooba T, Takizawa T, et al. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994; 71:73–81.
5. Busson P, Keryer C, Ooka T, Corbex M. EBV-associated nasopharyngeal carcinomas: from epidemiology to virus-targeting strategies. Trends Microbiol. 2004; 12:356–360.
6. Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008; 99:195–201.
7. Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000; 118:1031–1038.
8. Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002; 160:787–794.
9. Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976; 38:232–243.
10. Lertprasertsuke N, Tsutsumi Y. Gastric carcinoma with lymphoid stroma. Analysis using mucin histochemistry and immunohistochemistry. Virchows Arch A Pathol Anat Histopathol. 1989; 414:231–241.
11. Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y, et al. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990; 66:945–952.
12. Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994; 73:2239–2249.
13. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004; 22:664–670.
14. Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996; 27:20–27.
15. Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathol Res Pract. 2001; 197:395–400.
16. Park ES, Do IG, Park CK, Kang WK, Noh JH, Sohn TS, et al. Cyclooxygenase-2 is an independent prognostic factor in gastric carcinoma patients receiving adjuvant chemotherapy and is not associated with EBV infection. Clin Cancer Res. 2009; 15:291–298.
17. Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014; 63:236–243.
18. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209.
19. Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010; 139:84–92.
20. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009; 137:824–833.
21. Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009; 24:354–365.
22. Matsunou H, Konishi F, Hori H, Ikeda T, Sasaki K, Hirose Y, et al. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996; 77:1998–2004.
23. Arikawa J, Tokunaga M, Tashiro Y, Tanaka S, Sato E, Haraguchi K, et al. Epstein-Barr virus-positive multiple early gastric cancers and dysplastic lesions: a case report. Pathol Int. 1997; 47:730–734.
24. Chang MS, Lee HS, Kim HS, Kim SH, Choi SI, Lee BL, et al. Epstein-Barr virus and microsatellite instability in gastric carcinogenesis. J Pathol. 2003; 199:447–452.
25. Camargo MC, Koriyama C, Matsuo K, Kim WH, Herrera-Goepfert R, Liao LM, et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. Int J Cancer. 2014; 134:948–953.
26. Luo B, Wang Y, Wang XF, Gao Y, Huang BH, Zhao P. Correlation of Epstein-Barr virus and its encoded proteins with Helicobacter pylori and expression of c-met and c-myc in gastric carcinoma. World J Gastroenterol. 2006; 12:1842–1848.
27. Yanai H, Murakami T, Yoshiyama H, Takeuchi H, Nishikawa J, Nakamura H, et al. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J Clin Gastroenterol. 1999; 29:39–43.
28. Lima VP, de Lima MA, André AR, Ferreira MV, Barros MA, Rabenhorst SH. H pylori (CagA) and Epstein-Barr virus infection in gastric carcinomas: correlation with p53 mutation and c-Myc, Bcl-2 and Bax expression. World J Gastroenterol. 2008; 14:884–891.
29. Song HJ, Kim KM. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut Liver. 2011; 5:143–148.
30. Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus-negative carcinoma. Clin Cancer Res. 2004; 10:1698–1705.
31. Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996; 75:67–76.
32. Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein-Barr virus in gastric carcinoma. Am J Pathol. 1993; 143:1250–1254.
33. van Beek J, zur Hausen A, Snel SN, Berkhof J, Kranenbarg EK, van de Velde CJ, et al. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol. 2006; 30:59–65.
34. Kuzushima K, Nakamura S, Nakamura T, Yamamura Y, Yokoyama N, Fujita M, et al. Increased frequency of antigen-specific CD8(+) cytotoxic T lymphocytes infiltrating an Epstein-Barr virus-associated gastric carcinoma. J Clin Invest. 1999; 104:163–171.
35. Iwasaki Y, Chong JM, Hayashi Y, Ikeno R, Arai K, Kitamura M, et al. Establishment and characterization of a human Epstein-Barr virus-associated gastric carcinoma in SCID mice. J Virol. 1998; 72:8321–8326.
36. Chong JM, Sakuma K, Sudo M, Osawa T, Ohara E, Uozaki H, et al. Interleukin-1beta expression in human gastric carcinoma with Epstein-Barr virus infection. J Virol. 2002; 76:6825–6831.
37. Strong MJ, Xu G, Coco J, Baribault C, Vinay DS, Lacey MR, et al. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: implications for possible immune adjuvant therapy. PLoS Pathog. 2013; 9:e1003341.
38. Kim SY, Park C, Kim HJ, Park J, Hwang J, Kim JI, et al. Deregulation of immune response genes in patients with Epstein-Barrvirus-associated gastric cancer and outcomes. Gastroenterology. 2015; 148:137–147.
39. Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007; 56:739–745.
40. Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012; 1:1223–1225.
41. Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006; 108:19–24.
42. Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014; 96:284–291.
43. Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010; 116:1757–1766.
44. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012; 4:127ra37.
45. Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011; 117:2192–2201.
46. Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005; 11:2947–2953.
47. Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006; 66:3381–3385.
48. Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007; 56:1173–1182.
49. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007; 13:2151–2157.
50. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007; 104:3360–3365.
51. Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011; 6:e23621.
52. Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013; 1:54–63.
53. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014; 94:107–116.
54. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014; 50:1361–1369.
55. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014; 20:2773–2782.
56. Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014; 7:1–17.
57. Kim JW, Nam KH, Ahn SH, Park do J, Kim HH, Kim SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016; 19:42–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download