Abstract
Laparoscopic proximal gastrectomy (LPG) is theoretically a superior choice of minimally-invasive surgery and function-preserving surgery for the treatment of proximal early gastric cancer (EGC) over procedures such as laparoscopic total gastrectomy (LTG), open total gastrectomy (OTG) and open proximal gastrectomy (OPG). However, LPG and OPG are not popular surgical options due to three main concerns: the first, oncological safety; the second, functional benefits; and the third, anastomosis-related late complications (reflux symptoms and anastomotic stricture). Numerous recent studies have concluded that OPG and LPG present similar oncological safety profiles and improved functional benefits when compared with OTG and LTG. While OPG with modified esophagogastrostomy does not provide satisfactory results, OPG with modified esophagojejunostomy showed similar rates of anastomosis-related late complications when compared to OTG. At this stage, no standard reconstruction method post-LPG exists in the clinical setting. We recently showed that LPG with double tract reconstruction (DTR) is a superior choice over LTG for proximal EGC in terms of maintaining body weight and preventing anemia. However, as there is no definitive evidence in favor of LPG with DTR, a randomized clinical trial comparing LPG with DTR to LTG was recommended. This trial, the Korean Laparoscopic Gastrointestinal Surgery Study-05 (NCT01433861), is expected to assist surgeons in choice of surgical approach and strategy for patients with proximal EGC.
The epidemiology of gastric cancer has transformed over the last several decades in Korea. The incidence of early gastric cancer (EGC) has increased from 24.8% to 48.9% as a result of improved surveillance by means of the national cancer screening program in Korea. The incidence of proximal gastric cancer too has gradually increased from 5.3% to 14.0%.1
Recently, interest in minimally-invasive and function-preserving surgery for treating EGC has gained momentum among surgeons. In Korea, 26% of gastric cancer surgeries in 2009 were performed using laparoscopic procedures, an almost five-fold increase in use over a 5-year period.1 In recent decades, the oncological safety of minimally-invasive surgery for the treatment EGC has been established.2 As such, the main interest of minimally-invasive surgical techniques has shifted from technical and safety aspects towards function-preservation.
When function preservation or minimal invasiveness are taken into consideration, laparoscopic proximal gastrectomy (LPG) is the best theoretical treatment option for proximal EGC and outweighs open proximal gastrectomy (OPG), open total gastrectomy (OTG), and laparoscopic total gastrectomy (LTG). However, LPG is currently not a popular surgical choice and proximal gastrectomy (PG) (which entails both OPG and LPG) was performed in only 141 (1.0%) Korean patients in 2009.1 OPG too is not a standard surgical procedure existing rather as an alternative.
Before reviewing LPG, the current aversion towards OPG will be discussed in this article. The application of OPG is limited by the three main concerns: the first, oncological safety; the second, functional benefits, and the third, anastomosis-related late complications (reflux symptoms and anastomotic stricture).
Taking the above into consideration, LPG has been tested as an alternative treatment option for proximal EGC as most types of laparoscopic gastric cancer surgeries have improved safety profiles. The main concerns of LPG will also be reviewed in this article.
Radical gastrectomy involves negative margin gastrectomy and complete lymph node (LN) dissection of potential metastatic LNs. In OPG, negative margins are easily confirmed by frozen biopsy. However, the extent of the lymphadenectomy required can not always be determined either before or during the procedure, as the surgeon is not able to identify potential metastatic LNs during OPG.
However, several studies have provided guidelines for surgeons to determine the extent of lymphadenectomy required for proximal EGC. The rates of metastatic LNs in ECG are estimated at 10% to 20%. Kitamura et al.3 reported that LN metastasis along the lower part of the stomach is not observed in proximal gastric cancer confined to the muscularis propria. Kong et al.4 reported that proximal EGC metastasizes only to LN stations 2, 3, and 7. The Japanese Gastric Cancer Treatment Guidelines 2010 (version 3) recommends D1+ LN dissection for proximal EGC; LN stations 1, 2, 3a, 4sa, 4sb, 7, 8a, 9, and 11p.5
There have been several studies and meta-analyses comparing OPG to OTG. These concluded that the long-term overall survival rate is similar for proximal EGC when comparing OPG (88.7% to 98.5%) and OTG (87.6% to 99.2%).67891011121314
As the indication for OPG has recently been limited to proximal EGC, long-term overall survival rates have not been a major surgical concern.
An important consideration in OPG is the potential for remnant gastric cancer. The rate of remnant gastric cancer is higher after OPG (3.6% to 9.1%) than that seen after open distal gastrectomy (ODG) (0.4% to 2.5%).1015161718 Scheduled endoscopic follow-ups are the sole means of early detection and subsequent curative resection after OPG. Intubation of endoscopy following esophagogastrostomy (EG stomy) is not difficult, however, this can be a challenging procedure after esophagojejunostomy (EJ stomy), especially in cases with a longer interposed segment.1920 An interposed jejunum greater than 10 cm in length does present a challenge in terms of evaluating the remnant stomach.19 Therefore, the length of the interposed jejunum should be carefully chosen when considering endoscopic follow-up.
The benefits of nutrition status are controversial as several studies have shown that blood chemistry levels (such as protein, albumin, and cholesterol) related to the patient's postoperative nutritional status were higher after OPG when compared with OTG.21222324 Certain reports have demonstrated that total body weight loss was in fact decreased after OPG when compared with OTG.10122223252627 However, numerous long-term reports and a recent meta-analysis did conclude that the nutritional benefits and total body weight loss are similar when comparing OPG and OTG.711 As long-term follow-up data and a meta-analysis have shown similar nutritional benefits, the same is considered for the short-term postoperative period.
Several short-term follow-up studies have showed that postoperative hemoglobin levels are similar when comparing OPG and OTG. However, long term follow-up data collected 1 or 2 years post-procedure consistently show that hemoglobin levels are significantly higher after OPG when compared with OTG.610122425 Lower hemoglobin levels after OTG may be attributable to a vitamin B12 deficiency, a theoretical inevitability. However, a few studies have investigated vitamin B12 levels and the volume of supplementation administered while comparing OTG and OPG.25 In laparoscopic procedures, more than 80% of patients undergoing LTG required vitamin B12 supplementation. Despite supplementation, vitamin B12 levels are significantly lower after LTG when compared with LPG (Seoul National University Bundang Hospital [SNUBH] data, The 87th Annual meeting of the Japanese Gastric Cancer Association [JGCA]). This deficiency is believed to result in anemia after total gastrectomy, which occurs more frequently than that observed after PG.
In this review, quality of life was defined as all subjective symptoms with the exception of anastomosis-related symptoms (reflux and stenosis-related symptoms, which are reviewed below). Several reports used a standard questionnaire and concluded that subjective symptoms after single- meal intake were improved after OPG when compared with OTG.6252829 Recently, Takiguchi et al.26 evaluated subjective symptoms using a well-designed validated questionnaire and a post-gastrectomy syndrome assessment scale (PGSAS-45). Their data showed that OPG was significantly improved over OTG in terms of preventing body weight loss, the necessity for additional meals, diarrhea, and dumping.
OPG and OTG had sometimes similar nutrition statuses and body weight loss in the long-term follow up. However, OPG had a significantly higher hemoglobin level and better subjective symptoms when compared with OTG. Thus, OPG is recommended as a function-preserving procedure for proximal EGC.
Two types of post-OPG reconstruction methods are widely-known and classified according to the type of intestine involved: EG stomy and EJ stomy. Many modified EG and EJ stomy procedures have been tested for improved technical feasibility and prevention of anastomosis-related late complications (reflux esophagitis and anastomotic stenosis).
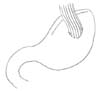
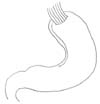
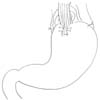
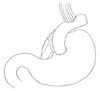
EG stomy is simpler than EJ stomy as it includes only one anastomosis. The feasibilities of many standard and modified EG stomy procedures (e.g., EG stomy with anti-reflux procedures) have been reported, including simple EG stomy (Fig. 1), reverse double stapling,4 lower esophageal sphincter preserving,30 gastric tube (Fig. 2),3132 gastropexy,33 fundoplication (Fig. 3),34 and acute angle EG stomy,28 among others. These EG stomy procedures had a lower surgical duration and decreased estimated blood loss (Table 1). However, these procedures could not demonstrate an acceptable incidence of anastomosis-related late complications. The rates of anastomosis-related late complications were significantly higher after OPG (27.4% to 67.4%) when compared with OTG (7.4% to 8.7%) in several studies comparing EG stomy after OPG with Roux-en-Y EJ stomy after OTG.791135
Many modified EJ stomy procedures have been tried in place of an EG stomy in an effort to prevent anastomosis-related late complications, including jejunal interposition (Fig. 4),610151619202136373839404142 jejunal pouch interposition,202529383943444546 double tract reconstruction (DTR) (Fig. 5),47 and more (Table 2). The incidence of anastomosis-related late complications was not significantly different when comparing modified EJ stomy after OPG (0% to 10.2%) and Rouxen-Y EJ stomy after OTG (1.8% to 8.7%).6113638 Thus, most modified EJ stomy procedures are considered a good reconstruction method in terms of preventing reflux esophagitis and anastomotic stenosis.
However, the modified EJ stomy after OPG does have certain disadvantages, including a lengthier surgical procedure time, increased estimated blood loss, and a higher rate of early complications.384142 In addition, jejunal interposition is associated with abdominal discomfort after meals, continuous gastric fullness, and hiccups between meals in the postoperative period; a result of the interposed segment which may disturb the passage of food.1740
To the best of our knowledge, no studies have been published with the aim of evaluating quality of life and comparing DTR and jejunal interposition or jejunal pouch interposition. However, DTR after OPG is the preferred anastomosis method over jejunal interposition or jejunal pouch interposition for reducing subjective symptoms, as DTR involves two food passages.
Uyama et al.48 first reported LPG in 1995 and there have since been several technical reports and small sample-sized case studies.4950515253545556575859606162 The main purpose of these articles was to evaluate the technical feasibility of the procedure, including acceptable procedure times, estimated blood loss, short-term complications, and anastomosis-related late complications. There are very few studies comparing LPG with OPG, and these found that LPG had a lengthier procedure time, decreased estimated blood loss, and similar complications rates when compared with OPG.63 To our knowledge, there is a single study comparing LPG with LTG64 and no reported prospective randomized clinical trials as yet.
A few of articles have reported on the oncological safety of LPG6064 and overall survival was not shown to be significantly different when comparing LPG with EG stomy and LTG with Rouxen-Y EJ stomy.64 While very few studies have discussed long-term oncological safety, it has been suggested that it would not be significantly different across LPG, LTG or OPG, as the indication for LPG is proximal EGC.
Functional outcomes have been discussed in a few studies, which have concluded that nutritional benefits were not significantly different when comparing LPG to EG stomy and LTG to Roux-en-Y stomy.64 However, 32% patients who underwent LPG with EG stomy had reflux symptoms exceeding Visick grade II. As reflux symptoms could affect the total nutritional status of LPG, the nutritional benefits of LPG are likely to be underestimated in this study.64 Reflux symptoms are reduced following DTR and hemoglobin levels were significantly higher in the first and second postoperative years after LPG when compared with LTG (SNUBH data, 87th Annual meeting of JGCA).
Two different reconstruction methods may be performed after LPG: EG and EJ stomy. Several modified laparoscopic EG and EJ stomy procedures have been evaluated for their technical feasibility and prevention of anastomosis-related late complications (Table 1, 2).49505152535455565758596061626364 However, all types of modified laparoscopic EG stomy procedures were shown to be unsatisfactory for the prevention of anastomosis-related late complications, or have been limited to a case series or technique reports involving laparoscopic modified EG stomy.4951525354575859606162 A recent article comparing LPG with EG stomy to LTG with Roux-en-Y concluded that the former was associated with an increased risk of reflux symptoms (LPG 32.0%, LTG 3.7%; P<0.001).64
Modified laparoscopic EJ stomy has been assessed by several groups5055565765 and a low incidence of anastomosis-related late complications has been observed. In addition, LPG with DTR was shown to have an acceptable duration time (mean procedure time: 108.7 minutes), acceptable estimated blood loss (estimated blood low: 120.4 ml), and a low incidence of anastomosis related late complications (reflux symptoms: 4.65%, anastomotic stenosis 4.65%).50 Therefore, LPG with DTR has the potential to be a standard reconstruction method for LPG although this is not decisive as it is based on a case-series.50
We recently analyzed and compared the clinical outcomes across LPG with DTR and LTG for proximal EGC. Anastomosis-related late complications were not significantly different when comparing LPG with DTR to LTG (SNUBH data, 87th Annual meeting of JGCA). While this was a retrospective study, the results were helpful in terms of processing prospective randomized clinical trials comparing LPG with DTR and LTG.
OPG showed a similar oncological safety profile and improved functional benefits when compared with OTG. Although OPG with modified EG stomy was not satisfactory, similar rates of anastomosis-related late complication were observed when comparing OPG with modified EJ stomy to OTG.
As minimally-invasive surgical techniques has become more widely used and accepted, its major aim has transformed from a focus on technical feasibility and oncological safety profiles to function preservation. Minimally-invasive surgery could be a standard procedure for EGC as it fulfills all patient requirements. Thus, LPG is a theoretically preferable treatment option over LTG, OTG, and OPG, as it is both minimally-invasive and function-preserving.
At this stage, no standard reconstruction method post-LPG exists in the clinical setting. We recently analyzed and compared the clinical outcomes of LPG with DTR and LTG for proximal EGC, and found that the former to be superior in terms of maintaining body weight and preventing anemia. A randomized clinical trial with the aim of comparing LPG with DTR to LTG was duly recommended and is now underway. This trial is named KLASS-05 (Korean Laparoscopic Gastrointestinal Surgery Study-05, NCT01433861) and results are expected to assist surgeons in the decision-making process when considering the surgical approach and strategy for patients with proximal EGC.
Figures and Tables
References
1. Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011; 11:69–77.
2. Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014; 32:627–633.
3. Kitamura K, Yamaguchi T, Nishida S, Yamamoto K, Ichikawa D, Okamoto K, et al. The operative indications for proximal gastrectomy in patients with gastric cancer in the upper third of the stomach. Surg Today. 1997; 27:993–998.
4. Kong SH, Kim JW, Lee HJ, Kim WH, Lee KU, Yang HK. Reverse double-stapling end-to-end esophagogastrostomy in proximal gastrectomy. Dig Surg. 2010; 27:170–174.
5. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
6. Zhao P, Xiao SM, Tang LC, Ding Z, Zhou X, Chen XD. Proximal gastrectomy with jejunal interposition and TGRY anastomosis for proximal gastric cancer. World J Gastroenterol. 2014; 20:8268–8273.
7. Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, et al. Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology. 2012; 59:633–640.
8. Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998; 123:127–130.
9. Pu YW, Gong W, Wu YY, Chen Q, He TF, Xing CG. Proximal gastrectomy versus total gastrectomy for proximal gastric carcinoma. A meta-analysis on postoperative complications, 5-year survival, and recurrence rate. Saudi Med J. 2013; 34:1223–1228.
10. Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg. 2013; 37:558–564.
11. An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008; 196:587–591.
12. Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, et al. Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer. 2014; 17:141–145.
13. Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004; 36:50–55.
14. Ikeguchi M, Kader A, Takaya S, Fukumoto Y, Osaki T, Saito H, et al. Prognosis of patients with gastric cancer who underwent proximal gastrectomy. Int Surg. 2012; 97:275–279.
15. Kikuchi S, Nemoto Y, Katada N, Sakuramoto S, Kobayashi N, Shimao H, et al. Results of follow-up endoscopy in patients who underwent proximal gastrectomy with jejunal interposition for gastric cancer. Hepatogastroenterology. 2007; 54:304–307.
16. Ohyama S, Tokunaga M, Hiki N, Fukunaga T, Fujisaki J, Seto Y, et al. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer. 2009; 12:88–94.
17. Hiki N, Nunobe S, Kubota T, Jiang X. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol. 2013; 20:2683–2692.
18. Nozaki I, Kurita A, Nasu J, Kubo Y, Aogi K, Tanada M, et al. Higher incidence of gastric remnant cancer after proximal than distal gastrectomy. Hepatogastroenterology. 2007; 54:1604–1608.
19. Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T, et al. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg. 2008; 32:1473–1477.
20. Iwata T, Kurita N, Ikemoto T, Nishioka M, Andoh T, Shimada M. Evaluation of reconstruction after proximal gastrectomy: prospective comparative study of jejunal interposition and jejunal pouch interposition. Hepatogastroenterology. 2006; 53:301–303.
21. Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014; 38:1100–1106.
22. Hinoshita E, Takahashi I, Onohara T, Nishizaki T, Matsusaka T, Wakasugi K, et al. The nutritional advantages of proximal gastrectomy for early gastric cancer. Hepatogastroenterology. 2001; 48:1513–1516.
23. Kondoh Y, Okamoto Y, Morita M, Nabeshima K, Nakamura K, Soeda J, et al. Clinical outcome of proximal gastrectomy in patients with early gastric cancer in the upper third of the stomach. Tokai J Exp Clin Med. 2007; 32:48–53.
24. Kim EM, Jeong HY, Lee ES, Moon HS, Sung JK, Kim SH, et al. Comparision between proximal gastrectomy and total gastrectomy in early gastric cancer. Korean J Gastroenterol. 2009; 54:212–219.
25. Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005; 29:1592–1599.
26. Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015; 18:407–416.
27. Nomura E, Lee SW, Tokuhara T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and type of reconstruction following open and laparoscopic proximal gastrectomy for gastric cancer. Hepatogastroenterology. 2012; 59:1677–1681.
28. Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg. 2013; 398:697–701.
29. Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: short- and long-term consequences. Am J Surg. 2012; 204:203–209.
30. Hirai T, Matsumoto H, Iki K, Hirabayashi Y, Kawabe Y, Ikeda M, et al. Lower esophageal sphincter- and vagus-preserving proximal partial gastrectomy for early cancer of the gastric cardia. Surg Today. 2006; 36:874–878.
31. Adachi Y, Katsuta T, Aramaki M, Morimoto A, Shiraishi N, Kitano S. Proximal gastrectomy and gastric tube reconstruction for early cancer of the gastric cardia. Dig Surg. 1999; 16:468–470.
32. Ronellenfitsch U, Najmeh S, Andalib A, Perera RM, Rousseau MC, Mulder DS, et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015; 22:772–779.
33. Kondoh Y, Ishii A, Ishizu K, Hanashi T, Okamoto Y, Morita M, et al. Esophagogastrostomy before proximal gastrectomy in patients with early gastric cancers in the upper third of the stomach. Tokai J Exp Clin Med. 2006; 31:146–149.
34. Ishigami S, Uenosono Y, Arigami T, Kurahara H, Okumura H, Matsumoto M, et al. Novel fundoplication for esophagogastrostomy after proximal gastrectomy. Hepatogastroenterology. 2013; 60:1814–1816.
35. Katsoulis IE, Robotis JF, Kouraklis G, Yannopoulos PA. What is the difference between proximal and total gastrectomy regarding postoperative bile reflux into the oesophagus? Dig Surg. 2006; 23:325–330.
36. Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg. 2010; 97:558–562.
37. Adachi Y, Inoue T, Hagino Y, Shiraishi N, Shimoda K, Kitano S. Surgical results of proximal gastrectomy for early-stage gastric cancer: jejunal interposition and gastric tube reconstruction. Gastric Cancer. 1999; 2:40–45.
38. Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, et al. Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery. 2014; 156:57–63.
39. Takagawa R, Kunisaki C, Kimura J, Makino H, Kosaka T, Ono HA, et al. A pilot study comparing jejunal pouch and jejunal interposition reconstruction after proximal gastrectomy. Dig Surg. 2010; 27:502–508.
40. Tokunaga M, Hiki N, Ohyama S, Nunobe S, Miki A, Fukunaga T, et al. Effects of reconstruction methods on a patient's quality of life after a proximal gastrectomy: subjective symptoms evaluation using questionnaire survey. Langenbecks Arch Surg. 2009; 394:637–641.
41. Ichikawa D, Ueshima Y, Shirono K, Kan K, Shioaki Y, Lee CJ, et al. Esophagogastrostomy reconstruction after limited proximal gastrectomy. Hepatogastroenterology. 2001; 48:1797–1801.
42. Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002; 26:1150–1154.
43. Tomita R, Fujisaki S, Tanjoh K, Fukuzawa M. A novel operative technique on proximal gastrectomy reconstructed by interposition of a jejunal J pouch with preservation of the vagal nerve and lower esophageal sphincter. Hepatogastroenterology. 2001; 48:1186–1191.
44. Yabusaki H, Nashimoto A, Matsuki A, Aizawa M. Evaluation of jejunal pouch interposition after proximal gastrectomy for early gastric cancer in the upper third of the stomach. Hepatogastroenterology. 2012; 59:2032–2036.
45. Kobayashi M, Araki K, Okamoto K, Okabayashi T, Akimori T, Sugimoto T. Anti-reflux pouch-esophagostomy after proximal gastrectomy with jejunal pouch interposition reconstruction. Hepatogastroenterology. 2007; 54:116–118.
46. Hoshikawa T, Denno R, Ura H, Yamaguchi K, Hirata K. Proximal gastrectomy and jejunal pouch interposition: evaluation of postoperative symptoms and gastrointestinal hormone secretion. Oncol Rep. 2001; 8:1293–1299.
47. Zhao Q, Li Y, Guo W, Zhang Z, Ma Z, Jiao Z. Clinical application of modified double tracks anastomosis in proximal gastrectomy. Am Surg. 2011; 77:1593–1599.
48. Uyama I, Ogiwara H, Takahara T, Kikuchi K, Iida S. Laparoscopic and minilaparotomy proximal gastrectomy and esophagogastrostomy: technique and case report. Surg Laparosc Endosc. 1995; 5:487–491.
49. Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Esophagogastrostomy using a circular stapler in laparoscopy-assisted proximal gastrectomy with an incision in the left abdomen. Langenbecks Arch Surg. 2012; 397:57–62.
50. Ahn SH, Jung do H, Son SY, Lee CM, Park do J, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. 2014; 17:562–570.
51. Kitano S, Adachi Y, Shiraishi N, Suematsu T, Bando T. Laparoscopic-assisted proximal gastrectomy for early gastric carcinomas. Surg Today. 1999; 29:389–391.
52. Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H, et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg. 2009; 209:344–351.
53. Okabe H, Obama K, Tanaka E, Tsunoda S, Akagami M, Sakai Y. Laparoscopic proximal gastrectomy with a hand-sewn esophago-gastric anastomosis using a knifeless endoscopic linear stapler. Gastric Cancer. 2013; 16:268–274.
54. Kim DJ, Lee JH, Kim W. Lower esophageal sphincter-preserving laparoscopy-assisted proximal gastrectomy in patients with early gastric cancer: a method for the prevention of reflux esophagitis. Gastric Cancer. 2013; 16:440–444.
55. Takayama T, Matsumoto S, Wakatsuki K, Tanaka T, Migita K, Ito M, et al. A novel laparoscopic procedure for treating proximal early gastric cancer: laparoscopy-assisted pylorus-preserving nearly total gastrectomy. Surg Today. 2014; 44:2332–2338.
56. Esquivel CM, Ampudia C, Fridman A, Moon R, Szomstein S, Rosenthal RJ. Technique and outcomes of laparoscopic-combined linear stapler and hand-sutured side-to-side esophagojejunostomy with Roux-en-Y reconstruction as a treatment modality in patients undergoing proximal gastrectomy for benign and malignant disease of the gastroesophageal junction. Surg Laparosc Endosc Percutan Tech. 2014; 24:89–93.
57. Yasuda A, Yasuda T, Imamoto H, Kato H, Nishiki K, Iwama M, et al. A newly modified esophagogastrostomy with a reliable angle of His by placing a gastric tube in the lower mediastinum in laparoscopy-assisted proximal gastrectomy. Gastric Cancer. 2014; DOI: 10.1007/s10120-014-0431-6. [epub].
58. Hosogi H, Yoshimura F, Yamaura T, Satoh S, Uyama I, Kanaya S. Esophagogastric tube reconstruction with stapled pseudofornix in laparoscopic proximal gastrectomy: a novel technique proposed for Siewert type II tumors. Langenbecks Arch Surg. 2014; 399:517–523.
59. Mochiki E, Fukuchi M, Ogata K, Ohno T, Ishida H, Kuwano H. Postoperative functional evaluation of gastric tube after laparoscopic proximal gastrectomy for gastric cancer. Anticancer Res. 2014; 34:4293–4298.
60. Takeuchi H, Oyama T, Kamiya S, Nakamura R, Takahashi T, Wada N, et al. Laparoscopy-assisted proximal gastrectomy with sentinel node mapping for early gastric cancer. World J Surg. 2011; 35:2463–2471.
61. Tonouchi H, Mohri Y, Tanaka K, Kobayashi M, Kusunoki M. Hemidouble stapling for esophagogastrostomy during laparoscopically assisted proximal gastrectomy. Surg Laparosc Endosc Percutan Tech. 2006; 16:242–244.
62. Aihara R, Mochiki E, Ohno T, Yanai M, Toyomasu Y, Ogata K, et al. Laparoscopy-assisted proximal gastrectomy with gastric tube reconstruction for early gastric cancer. Surg Endosc. 2010; 24:2343–2348.
63. Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc. 2013; 27:146–153.
64. Ahn SH, Lee JH, Park do J, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. 2013; 16:282–289.
65. Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, et al. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol. 2014; 12:20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download