Abstract
It is well known that gastrectomy with curative intent is the best way to improve outcomes of patients with remnant gastric cancer. Recently,several investigators reported their experiences with laparoscopic gastrectomy of remnant gastric cancer. We report the case of an 83-year-old female patient who was diagnosed with remnant gastric cancer with obstruction. She underwent an entirely laparoscopic distal gastrectomy with colectomy because of direct invasion of the transverse colon. The operation time was 200 minutes. There were no postoperative complications. The pathologic stage was T4b (transverse colon) N0M0. Our experience suggests that laparoscopic surgerycould be an effective method to improve the surgical outcomes of remnant gastric cancer patients.
Although the incidence of remnant gastric cancer has been reported to be low,1 it is expected to increase with more early diagnoses because of regular follow-up for cancer.2 Remnant gastric cancer is defined as carcinoma arising from the remnant stomach regardless of whether prior disease was benign or malignant. Traditionally, remnant gastric cancer has been recognized as an advanced state with poor prognosis.3 In spite of this, it is well known that complete resection (R0) with lymph node dissection is the most critical determinant to improve overall survival.
It is well known that surgical procedures for remnant gastric cancer are highly invasive because of severe intraperitoneal adhesions and direct invasion of disease into surrounding structures. Despite these difficulties, several investigators have erported their experiences regarding surgical outcomes of laparoscopic surgeries for remnant gastric cancers.45 Thus, we also report the case of an 83-year-old female who underwent an entirely laparoscopic resection of a remnant gastric cancer with gastric outlet obstruction and transverse colon invasion.
An 83-year-old woman was referred to the Hanyang University Guri Hospital for the treatment of a remnant gastric cancer. She had poor oral intake and repetitive vomiting a month ago and had lost more than 10 kg in the past six months. She had a history of open gastrectomy for gastric cancer 28 years ago. Although she had no specific underlying disease, her American Society of Anesthesiologists (ASA) score was graded as an ASA score of three because of old age and weight loss. Her body mass index (kg/m2) was 20.7 (height of 139 cm and weight of 49 kg).
Laboratory results revealed anemia (11.1 g/dl)6 and hypoalbuminemia (3.3 g/dl).7 Preoperative imaging studies, including abdominal-pelvic computed tomography (CT) and positron emission tomography CT scan, suggested T4aN1M0 as the clinical stage when accounting for evidence of serosa exposure. Levin tube placement and total parenteral nutrition (TPN) therapy were continued for six days prior to surgery. The Levin tube was removed in the operating room, and TPN was continued until the patient began a soft diet after surgery.
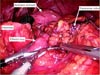
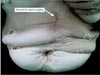
Despite her advanced preoperative clinical stage, this patient underwent laparoscopic gastrectomy at the request of her caretakers. The patient was placed in the reverse Trendelenburg position. After the initial trocar had been placed, lysis of peritoneal adhesions performed throughout the abdominal cavity. After completion of adhesiolysis, we were able to confirm the type of prior reconstruction (Billroth II without jejunojejunostomy). Dissection was begun by dividing the lesser curvature of the remnant stomach. However, it was impossible to dissect the posterior side and the greater curvature side of the remnant stomach because of the cancer's direct invasion of the transverse colon (Fig. 1). Therefore, a colectomy including resection of the tumor was performed using a laparoscopic linear stapler (ECHELON FLEX™ ENDOPATH® Stapler 60; Ciudad Juarez, Chihuahua, Mexico). Small bowel resections using the laparoscopic linear stapler were conducted in both the efferent and afferent loop. Next, the transverse mesocolon and the posterior side of the remnant stomach were dissected in order. All lymph nodes surrounding the splenic artery and greater curvature areas were dissected. After mobilization of the remnant stomach, distal gastrectomy with clear resection margins was performed using the laparoscopic linear stapler. We did not perform a total gastrectomy because the length of the proximal margin was grossly adequate. The specimen extraction was conducted through the umbilical port by extending the incision (Fig. 2). After remaking the pneumoperitoneum, the side-to-side anastomosis of the colon was performed using a laparoscopic linear stapler (Fig. 3A, B). The jejunojejunostomy was then created before reconstructing a gastrojejunostomy because of the short length of the afferent loop. Finally, the Roux-en-Y gastrojejunostomy was reconstructed (Fig. 3C, D). The operation time was 200 minutes (from skin incision to skin closure). The 100 ml of estimated blood loss was calculated by an anesthesiologist. A soft diet was commenced on the seventh postoperative day. The patient was discharged from the hospital on the thirteenth postoperative dya. There were no postoperative complications during the recovery period. Fig. 4 shows the appearance of the entire abdomen after surgery. Ultimately, the patient and her caretakers decided to discontinue the adjuvant chemotherapy because of the patient's advanced age.
From the pathology results, the tumor was noted to directly invade the transverse colon and jejunum. The tumor size was 7 cm. The proximal and distal resection margins were 2.5 cm and 4.5 cm, respectively. The number of retrieved lymph nodes was 24, and there were no metastatic lymph nodes.
Surgical resections of remnant gastric cancers are more invasive than resections of primary stomach cancers due to the presence of adhesions and direct invasion. Previously, there has been a report that the severity of adhesions and direct invasion is greater in patients who underwent an initial operation for gastric cancer due to previous lymph node dissections.8 Additionally, many patients with remnant gastric cancer are advanced in age and malnourished; this was true of our patient who presented at an advanced age with hypoalbuminemia. Despite the difficulties of the surgical procedure and the generally poor preoperative condition of patients, it is well known that a curative gastrectomy with lymph node dissection is the best way to improve overall survival.
Laparoscopic gastrectomy has become one of the most widely used surgical approaches in the treatment of early gastric cancer because of benefits such as less pain and faster recovery.910 Although there is no evidence regarding the oncologic safety of laparoscopic surgery for advanced (T2-T4) remnant gastric cancer, several investigators have suggested that laparoscopic surgery is feasible and safe for remnant gastric cancer.45 Moreover, as laparoscopic devices and surgical techniques are refined, mnay investigators are attempting to apply laparoscopic approaches to difficult cases such as adhesive ileus and complicated peptic ulcer disease.1112 The present author could also learn techniques for laparoscopic dissections of complicated peptic ulcer diseaes.11
We propose that laparoscopic surgery can minimize the damage to the patient during and after surgery. Especially in older patients and patients with malnutrition, we are convinced that laparoscopic surgery can improve early surgical outcome measures such as operation time and volume of blood loss. However, we believe that successful completion of the learning period required for laparoscopic gastrectomy is a prerequisite to achieve improved surgical outcomes. In particular, careful attention is needed in the process of adhesiolysis and the understanding of anatomical deterioration
Figures and Tables
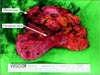 | Fig. 2The extracted specimen showed direct invasion of remnant gastric cancer into efferent loop and transverse colon. |
References
1. Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007; 94:92–95.
2. Pointner R, Schwab G, Königsrainer A, Bodner E, Schmid KW. Early cancer of the gastric remnant. Gut. 1988; 29:298–301.
3. Thorban S, Böttcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000; 231:188–194.
4. Kwon IG, Cho I, Guner A, Choi YY, Shin HB, Kim HI, et al. Minimally invasive surgery for remnant gastric cancer: a comparison with open surgery. Surg Endosc. 2014; 28:2452–2458.
5. Nagai E, Nakata K, Ohuchida K, Miyasaka Y, Shimizu S, Tanaka M. Laparoscopic total gastrectomy for remnant gastric cancer: feasibility study. Surg Endosc. 2014; 28:289–296.
6. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010; 16:Suppl Issues. S59–S66.
7. Detsky AS, Baker JP, O'Rourke K, Johnston N, Whitwell J, Mendelson RA, et al. Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 1987; 11:440–446.
8. Fujita J, Kurokawa Y, Sugimoto T, Miyashiro I, Iijima S, Kimura Y, et al. Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer. 2012; 15:42–48.
9. Russell MC, Mansfield PF. Surgical approaches to gastric cancer. J Surg Oncol. 2013; 107:250–258.
10. Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, et al. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011; 25:1076–1082.
11. Kim MG. Laparoscopic surgery for perforated duodenal ulcer disease: analysis of 70 consecutive cases from a single surgeon. Surg Laparosc Endosc Percutan Tech. 2015; 25:331–336.
12. Guo J, Liang Z, Zhang H, Yang C, Pu J, Mei H, et al. Laparoscopic versus open orchiopexy for non-palpable undescended testes in children: a systemic review and meta-analysis. Pediatr Surg Int. 2011; 27:943–952.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download