Abstract
Purpose
Gastric submucosal tumors (SMTs) located very close to the esophagogastric junction (EGJ) are a challenge for gastric surgeons. Therefore, this study reports on the experience of using endoscopic and laparoscopic full-thickness resection (ELFR) with laparoscopic two-layer suturing in such tumors.
Materials and Methods
Six patients with gastric SMTs very close to the EGJ underwent ELFR with laparoscopic two-layer suturing at Kyungpook National University Medical Center. With the patient under general anesthesia, the lesser curvature and posterior aspect adjacent to the EGJ were meticulously dissected and visualized using a laparoscopic approach. A partially circumferential full-thickness incision at the distal margin of the tumor was then made using an endoscopic approach under laparoscopic guidance. The SMT was resected using laparoscopic ultrasonic shears, and the gastric wall was closed using two-layer suturing. Thereafter, the patency and any leakage were checked through endoscopy.
Results
All the ELFR procedures with laparoscopic two-layer suturing were performed successfully without an open conversion. The mean operation time was 139.2±30.9 minutes and the blood loss was too minimal to be measured. The tumors from four patients were leiomyomas, while the tumors from the other two patients were gastrointestinal stromal tumors with clear resection margins. All the patients started oral intake on the third postoperative day. There was no morbidity or mortality. The mean hospital stay was 7.7±0.8 days.
Gastric submucosal tumors (SMTs) are not rare findings during an upper gastrointestinal endoscopy, with an incidence of 0.36% in routine examinations,1 and show a wide variety in both the origins of the tumor and histological spectra (from benign to malignant, such as a gastrointestinal stromal tumor [GIST]),2 and the locations (from the esophagogastric junction [EGJ] to the pylorus). Because of the difficulty in assessing the histological characteristics preoperatively, the entire resection of the tumor with a negative macroscopic and microscopic margin is indicated in most cases.3 However, with the wide acceptance of laparoscopic surgery, laparoscopic wedge resection is now considered safe and advantageous for relatively small- and medium-sized gastric SMTs.456 Notwithstanding, an endophytic gastric SMT located very close to the EGJ is still a challenge for laparoscopic gastric surgeons, making it difficult to avoid a gastrectomy due to the risk of stricture or stenosis.7
Accordingly, this study introduces the use of endoscopic and laparoscopic full-thickness resection (ELFR) with laparoscopic two-layer suturing as a safe treatment method8 that reduces unintended resection of the normal gastric wall and provides an adequate surgical margin.
Between March 2012 and February 2013, eight patients with gastric SMTs very close to the EGJ underwent surgery at the Kyungpook National University Medical Center in Daegu, South Korea. The preoperative workup for all the patients included history taking, physical examination, blood test, electrocardiogram, chest X-ray, gastrointestinal endoscopy with or without endoscopic ultrasonography, and computerized tomography of the abdomen and pelvis.
Among these patients, two were excluded because the distance from the Z-line to the proximal margin of the tumors was more than 2 cm.
Thus, six patients in whom the tumors were located very close to the EGJ (less than 2 cm from the Z-line) with an intragastric growth pattern and with a tumor size of 2 cm or greater were included. The preoperative endoscopic findings for the patients are presented in Fig. 1. After discharge, follow-up endoscopy was performed 6 months later.
Under general anesthesia, the patient was placed in a supine position. The operator and laparoscopist both stood on the right side of the patient, while the surgical assistant stood on the left side. An 11-mm-sized camera port was inserted into an inferior umbilical incision using an open technique, and additional 12-and 5-mm ports were inserted into the right side of the abdomen. In addition, another 5-mm port was inserted into the left side of the abdomen for the endoscopy assistant. After establhising the pneumoperitoneum at a pressure of 12 mmHg, intestinal clamps were placed across the proximal jejunum to prevent any gaseous dilatation of the distal bowel. Next, meticulous dissection of the lesser curvature adjacent to the EGJ was conducted using ultrasonic shears (Harmonic Ace®; Ethicon Endosurgery, Cincinnati, OH, USA). The initial dissection was made minimally to reduce the risk of poor blood supply to the area of the gastric wall closure, and further dissection was carried out if needed during the operation. The laparoscopic procedure is presented in Fig. 2, and the endoscopic procedure is presented in Fig. 3.
After dissecting the lesser curvature, intraluminal endoscopy (Q180®; Olympus, Tokyo, Japan) was used to identify the location of the tumor. A submucosal injection of a 0.9% saline solution mixed with epinephrine was administered, and a partially circumferential full-thickness incision of the gastric wall around the tumor was made. During the full-thickness incision by the endoscopist, the operator monitored the procedure under the laparoscopic view, and gauze was used as padding in the area of penetration to prevent any injury to adjacent structures caused by the endoscopic incision using a needle-knife. As a rule, endoscopic incision was made in the gastric wall distal to the mtuor and further circumferential incision was made in the proximal direction using an insulation-tipped diathermic electrosurgical knife. If the partially circumferential full-thickness incision was sufficient for the operator to hold and evert the tissue adjacent to the tumor, the tumor resection was completed laparoscopically using ultrasonic shears and the endoscope was withdrawn to the level of the lower part of the esophagus. After resection, the specimen was immediately placed in a plastic bag, and the resection margin and esophageal opening were identified. The gastric wall closure was made perpendicular to the longitudinal axis of the stomach. First, the gastric mucosa layer and submucosal layer were approximated with a synthetic absorbable sutuer (3-0 Vicryl®; Ethicon, Somerville, NJ, USA) using a continuous locking suture technique. The second-layer suture then approximated the seromuscular layer with a barbed unidirectional absorbable suture (V-Loc®; Covidien, Mansfield, MA, USA) using a continuous running suture technique. After closing the gastric wall defect, intraluminal endoscopic examination and simultaneous extraluminal laparoscopy were performed to identify any air leaks, bleeding, or stenosis. The operative field was irrigate dwith copious warm saline and the left subphrenic area was swabbed with dry gauze to remove any remnant fluid and thus reduce the risk of intraperitoneal infection. Peritoneal drain was not placed in any of the patients. The specimen was retrieved through the left 12-mm port with or without a 1- to 2-cm extension.
The patient characteristics are presented in Table 1.
During the operation, the blood loss was minimal and no transfusion was necessary. The mean operation time and anesthesia time were 139.2±30.9 and 182.8±34.2 minutes, respectively. The mean size of the specimens and tumors was 4.3±1.1 and 3.1±0.7 cm, respectively. None of the six patients underwent open conversion. All the patients started oral intake on the third postoperative day, and the mean postoperative hospital stay was 7.7±0.8 days. No complications were experienced. Four of the six tumors were leiomyomas, and the remaining two were low-risk GISTs. All the resected tumors showed clear resection margins (Table 2).
All of the six patients underwent follow-up endoscopy 6 months after discharge, and none showed stenosis, specific symptoms of reflux, or swallowing difficulty. The follow-up endoscopic findings are presented in Fig. 4.
Surgical resection with a tumor-free margin is the first choice of treatment for gastric SMTs towing to the difficulties and limitations of a definitive preoperative diagnosis.3 In addition, the advantages of less invasiveness, earlier recovery with a better postoperative quality of life, better cosmesis, and rapid advances in laparoscopic techniques and instruments have resulted in laparoscopic surgery being widely accepted as standard treatment for small- and medium-sized gastric SMTs.
Thus, in the case of GISTs, the consensus meeting of the European Society for Medical Oncology in 2004 suggested that laparoscopic resection should be indicated for tumors that are 2 cm or smaller,9 plus with the expansion of laparoscopic resection, the National Comprehensive Cancer Network Task Force Report: Update on the Management of Patients with GISTs and the Clinical Practice Guidelines for GISTs in Japan suggested that laparoscopic resection is feasible and can be safely performed for GISTs or SMTs that are 5 cm or smaller when applying the principles of surgical treatment of cancer.1011
Yet, gastric SMTs very close to the EGJ remain a challenge for laparoscopic gastric surgeons because of the narrow space, the absence of any redundant gastric wall for the laparoscopic linear stapler, and the possibility of postoperative deformity, stenosis, and leakage. Some have reported that gastric resection is unavoidable when the tumor is located near the EGJ or pylorus,7 but major gastric resection might be too invasive for a gastric SMT that may be a benign tumor. Therefore, various stomach-preserving treatments, including laparoscopic treatments, have already been introduced for gastric SMTs located close to the EGJ. Song et al.12 reported 10 cases of exogastric and transgastric resection using laparoscopic linear staplers; however, they found that multiple staples were needed in the case of an exogastric resection to prevent deformity, while a transgastric resection required more staples to close the gastrotomy. Hiki et al.13 reported three cases of tumors in the upper stomach and one case near the EGJ treated by laparoscopic and endoscopic cooperative surgery. For the EGJ tumor, they performed endoscopic incision of the mucosa and submucosa and laparoscopic dissection of the seromuscular layers. In addition, Hwang et al.14 performed transgastric enucleation, whereas Shim et al.15 performed intragastric enucleation. There have also been reports of successful endoscopic submucosal dissection and enucleation.1617 Yet, enucleation (shell-out procedure) is not recommended when a GIST is suspected.91118 For gastric SMTs without a definite preoperative pathological diagnosis, the possibility of a GIST should always be considered and the surgical principles of GIST treatment should be applied.
Tagaya et al.19 reported six cases of laparoscopic intragastric stapled resections; however, their procedure required specialized balloon-type trocars and multiple incisions into the anterior wall of the stomach that needed to be closed. Moreover, this technique cannot be applied to tumors on the anterior wall of the stomach. Sakamoto et al.20 reported five cases of exogastric resection, yet they performed a proximal gastrectomy when the tumor was less than 2 cm away from the EGJ.
The ELFR procedure used in this study was previously reported by Abe et al.8 for the treatment of early gastric cancer21 and was used for the treatment of gastric SMTs. In the present study, the authors applied the same technique for the treatment of endophytic gastric SMTs very close to the EGJ based on the following two reasons: (1) in the case of an endophytic gastric SMT close to the EGJ, laparoscopic localization of the tumor and deciding on the proper incision line are both difficult, thus, an endoscopic assistant is necessary; and (2) laparoscopic suturing is a good substitute for stapling in the narrow and complication-producing area of the EGJ to avoid both complication and major resection of the stomach.
Endoscopy has the advantage of being able to localize an intraluminal gastric tumor, and the application of an energy device enables an endoscope to perform various intraluminal surgical procedures. In addition, endoscopy by a well-disciplined endoscopist is more accurate than laparoscopy in targeting the peritumoral resection margin, thereby avoiding tumor rupture and guaranteeing a tumor-free resection margin. Thus, after making a partially circumferential full-thickness incision using an endoscope, the tumor can be everted and resected under direct visualization using a laparoscope. Thus, using the mersit of both endoscopy and laparoscopy, ELFR is safe and effective for a gastric SMT close to the EGJ.
Notwithstanding, following the resection of a gastric SMT close to the EGJ, there is a significant discrepancy in the lengths of the proximal and distal parts of the resection margin. Furthermore, it is difficult to apply a laparoscopic linear stapler to overcome the discrepancy in the length and diameter between the margin on the esophageal side and the margin on the gastric side, and even if it is possible, several staples are needed to make a round tube.
Thus, to overcome this discrepancy, laparoscopic two-layer suturing is safe, technically feasible, and relatively inexpensive. First, the mucosal and submucosal layers are approximated using a continuous locking suture, and the seromuscular coats are then approximated using a continuous running suture. The strength and elasticity of the submucosal layer allow the resection margins to be. The closure of the muscular layer then helps to reinforce the gastric wall closure and inversion of the gastric mucosa. However, in these procedures, a basic skill for laparoscopic suturing is a prerequisite for surgeons.
After 6 months, all the patients had no specific symptoms of swallowing difficulty or gastroesophageal reflux and follow-up endoscopic finding showed no reflux esophagitis or stenosis of the EGJ.
In conclusion, this study used ELFR and laparoscopic two-layer suturing for the treatment of gastric SMTs located close to the EGJ with favorable postoperative outcomes. Therefore, this technique should be considered as one of the solutions for the treatment of endophytic gastric SMTs that are very close to the EGJ.
Figures and Tables
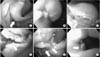
Fig. 2
Laparoscopic procedures. Application of intestinal clamps to the proximal jejunum (A), dissection of the lesser curvature of the esophagogastric junction (arrow) (B), perforation of the gastric wall using a needle-knife (arrow) (C), partially circumferential full-thickness incision using an insulation-tipped knife (arrow) (D), laparoscopic resection of the tumor using ultrasonic shears (E), identification of the esophageal opening (arrow) (F), closure of the mucosa and submucosa (G), and closure of the seromuscular layer (H).

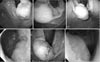
Fig. 3
Endoscopic procedures. Submucosal tumor (arrow) close to the esophagogastric junction before resection (A), submucosal injection of 0.9% saline solution mixed with epinephrine (B), formation of submucosal incision at the distal margin (arrow) of the tumor using a needle-knife (C), partially circumferential full-thickness incision of the gastric wall using a diathermic electrosurgical (insulation-tipped) knife, visible peritoneal cavity, and padded gauze (arrow) (D), and after resection and closure, showing a well-approximated gastric mucosa (arrows) (E, F).
Acknowledgments
This research was partly supported by the promoting project for regional advanced components and materials (A000600028) of the Ministry of Knowledge Economy, Korea.
References
1. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991; 5:20–23.
2. Ponsaing LG, Kiss K, Hansen MB. Classification of submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007; 13:3311–3315.
3. Berindoague R, Targarona EM, Feliu X, Artigas V, Balagué C, Aldeano A, et al. Laparoscopic resection of clinically suspected gastric stromal tumors. Surg Innov. 2006; 13:231–237.
4. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006; 243:738–745. discussion 745-747
5. Sexton JA, Pierce RA, Halpin VJ, Eagon JC, Hawkins WG, Linehan DC, et al. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc. 2008; 22:2583–2587.
6. Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010; 147:516–520.
7. Choi YB, Oh ST. Laparoscopy in the management of gastric submucosal tumors. Surg Endosc. 2000; 14:741–745.
8. Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, et al. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009; 23:1908–1913.
9. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.
10. Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8:Suppl 2. S1–S41. quiz S42-S44
11. Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. GIST Guideline Subcommittee. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–430.
12. Song KY, Kim SN, Park CH. Tailored-approach of laparoscopic wedge resection for treatment of submucosal tumor near the esophagogastric junction. Surg Endosc. 2007; 21:2272–2276.
13. Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008; 22:1729–1735.
14. Hwang SH, Park do J, Kim YH, Lee KH, Lee HS, Kim HH, et al. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc. 2009; 23:1980–1987.
15. Shim JH, Lee HH, Yoo HM, Jeon HM, Park CH, Kim JG, et al. Intragastric approach for submucosal tumors located near the Z-line: a hybrid laparoscopic and endoscopic technique. J Surg Oncol. 2011; 104:312–315.
16. Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012; 75:1153–1158.
17. Katoh T, Itoh Y, Mohri T, Suzuki H. Endoscopic enucleation of gastrointestinal stromal tumors of the stomach: report of five cases. World J Gastroenterol. 2008; 14:2609–2611.
18. Kang YK, Kim KM, Sohn T, Choi D, Kang HJ, Ryu MH, et al. Korean GIST Study Group. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. J Korean Med Sci. 2010; 25:1543–1552.
19. Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002; 16:177–179.
20. Sakamoto Y, Sakaguchi Y, Akimoto H, Chinen Y, Kojo M, Sugiyama M, et al. Safe laparoscopic resection of a gastric gastrointestinal stromal tumor close to the esophagogastric junction. Surg Today. 2012; 42:708–711.
21. Abe N, Mori T, Takeuchi H, Ueki H, Yanagida O, Masaki T, et al. Successful treatment of early stage gastric cancer by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Gastrointest Endosc. 2008; 68:1220–1224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download