Abstract
Sarcomatoid carcinoma of the small intestine is rare, and only 30 cases have been reported to date. This disease generally exhibits a very poor prognosis. Here we report the case of a 67-year-old man with a sarcomatoid carcinoma in the jejunum, who was hospitalized for diarrhea, fever, nausea, and vomiting. The tumor was located at the jejunum and had a large round shape with geographic necrosis. It involved the entire wall of the small intestine and had directly invaded the neighboring sigmoid colon. Both lobes of the liver had multiple metastases. The patient underwent surgical resection of the jejunum. On immunohistochemical analysis, the tumor was positive for epithelial and mesenchymal markers. The patient died from rapid progression of the liver metastases 6 weeks after the surgery.
Sarcomatoid carcinoma (SCA) is a rare biphasic tumor composed of malignant epithelial and mesenchymal cells. Only 30 cases of SCA of the small intestine have been reported to date.12 SCA is diagnosed on the basis of immunohistochemical staining as well as the histological identification of a mixture of carcinomatous and sarcomatous components. The clinical presentation of SCA of the small intestine includes abdominal pain, anemia, obstruction, and gastrointestinal bleeding.23 There is a lack of clinical evidence regarding the treatment of SCA; however, surgical resection is the optimal therapeutic approach at present.14 The effects of chemotherapy and radiotherapy have not yet been determined. The average life expectancy after a diagnosis of SCA is a few months because of the tumor's metastatic nature. SCA is known to be more malignant than adenocarcinoma of the small intestine.5 We report of a 67-year-old man with SCA in the jejunum and multiple liver metastases.
The patient was 67 years old and had no specific underlying disease. The patient lost 10 kg in the last month. Twenty days before hospitalization, the patient experienced continuous watery diarrhea and fever, and he was diagnosed with enterocolitis at a local hospital. The patient's symptoms were mitigated with medication, but he developed new symptoms including nausea and vomiting. An abdominal computed tomography (CT) scan taken at a local hospital revealed the possibility of cancer of the small intestine with metastasis to the liver. The patient was admitted to our hospital's internal medicine department.
At the time of hospitalization, the patient had a fever of 38℃ and appeared to be chronically ill. A physical examination revealed a soft abdomen without tenderness or rebound tenderness. A mass was palpable in the lower abdominal cavity. At the time of hospitalization, his diarrhea had subsided.
The patient's abdominal CT scan revealed a 67×61×76 mm3 large mass in the pelvic ileal loop, including a large ulcer and an area of necrotic tissue. Both lobes of the liver had multiple necrotic masses. Based on the abdominal CT scan, there was a high possibility of a malignant gastrointestinal stromal tumor (GIST) in the small intestine or an adenocarcinoma with liver metastasis. The blood test results for hemoglobin (7.9 g/dl), hematocrit (24.1%), iron (10 µg/dl), and unsaturated iron binding capacity (142 µg/dl) were indicative of iron deficiency anemia. In addition to fever, the patient's white blood cell count and C-reactive protein levels were elevated to 17,800/µl (71.1% neutrophils) and 12.63 mg/dl, respectively. However, chest radiography, abdominal CT, urine analysis, and physical examination revealed no apparent cause of the fever. Alpha-fetoprotein and carcinoembryonic antigen levels were within normal ranges, and other tumor markers were not tested.
Before surgery, ultrasonography-guided liver needle biopsy was performed to obtain an accurate diagnosis. The results were indicative of a poorly differentiated carcinoma. Immunohistochemical staining of the specimen revealed the possibility of SCA.
The advanced stage of the patient did not allow for curative resection, and there was a high possibility of cancer bleeding as well as intestinal obstruction and other complications. As a result, the patient was referred to the department of surgery, where he underwent palliative small intestine segmental resection and anterior resection.
A 10×6-cm2 round mass with geographic necrosis was located at the jejunum (260 cm from the ileocecal valve). The mass involved the entire wall of the small intestine and directly invaded the neighboring sigmoid colon. Both lobes of the liver appeared to have multiple metastases. The cancer did not appear to have metastasized to any other organs in the abdominal cavity except for the liver. Fourteen mesenteric lymph nodes were resected, and of those, metastasis (TNM stage pT4N1M1) was confirmed in two lymph nodes.
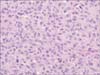
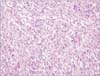
Microscopically, the tumor was composed of two cell components, namely ovoid to anaplastic tumor cells and spindle malignant cells. The ovoid to anaplastic tumor cells forming solid sheets without intracytoplasmic mucin globules represented the carcinomatous portion of the tumor (Fig. 1), whereas the spindle malignant cells in a fascicular arrangement represented the sarcomatous portion of the tumor (Fig. 2). The two components were intermixed, and both cell types showed vesicular chromatin, prominent nucleoli, and eosinophilic cytoplasm.
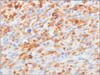
Immunohistochemical staining revealed a diffuse and strong positive reaction for vimentin, a positive reaction for Pan-CK (Fig. 3), and a focal positive reaction for c-kit (CD117). By contrast, CK20, CDX2, CD3, CD30, CEA, desmin, myoD1, and S-100 all appeared to be negative. A negative reaction was noted for DOG-1 (Fig. 4), which is usually positive in GISTs. The pathological test results were indicative of SCA of the jejunum.
The patient was discharged on the 16th day after surgery with no postoperative complications. The patient was scheduled to undergo palliative chemotherapy.
Small intestinal malignancies are rare and comprise less than 5% of all gastrointestinal cancers.6 Adenocarcinomas are the most common malignancy of this type, accounting for 30% to 50% of all small intestinal malignancies. Following adenocarcinomas, the next most common tumors are carcinoid tumors, stromal tumors, and lymphomas.6 SCA is a rare disease that normally occurs in the stomach, gallbladder, and esophagus.7 SCA of the small intestine is even rarer, as only <30 cases have been reported to date.12 It normally occurs in elderly patients at a mean age of 57 years, with a male to female ratio of 1.5:1.0. 1 In the small intestine, SCA primarily occurs in the ileum, followed by the jejunum and duodenum.1
Histologically, SCA displays carcinomatous and sarcomatous features. In SCA with biphasic patterns, epithelium-like and mesenchymal-like cells appear mixed, but in SCA with monophasic patterns, mesenchymal components comprise the majority of the tumor, with few to no epithelial components.1 Grossly, SCA is polypoid or endophytic, and it is accompanied by central ulceration.7 In many cases, the tumor is necrotic or hemorrhagic.
SCA has been described by various names, including sarcomatoid carcinosarcoma, pleomorphic carcinoma, undifferentiated carcinoma, pleomorphic giant cell carcinoma, anaplastic giant cell carcinoma, and giant cell carcinoma. Currently, SCA has been adopted as the uniform name for this malignancy.
The risk factors of SCA are unknown, but in certain publications, a correlation with long-standing regional enteritis has been referenced.89 The symptoms of SCA of the small intestine include abdominal pain, anemia, obstruction, and gastrointestinal bleeding. 23
Diseases to be considered in the differential diagnosis of SCA include leiomyosarcoma, GIST, schwannoma, and epithelioid angiosarcoma. To confirm a diagnosis of SCA, the sarcomatous component must display epithelial differentiation. It is difficult to establish this finding using conventional hematoxylin and eosin staining, and it is crucial that immunohistochemical markers are examined. Leiomyosarcoma is positive for muscle-specific actin and desmin, whereas SCA is negative. Positivity for cytokeratin and negativity for c-kit, CD34, and DOG-1 exclude the possibility of GISTs and angiosarcoma. For the differential diagnosis, it is useful to verify the c-kit immunostaining pattern because c-kit expression is usually diffuse and strong in GISTs, whereas it is weak and focal in carcinoma. Negativity for S-100 excludes the possibility of schwannoma. SCA is usually positive for cytokeratin and vimentin, and negative for desmin, SMA, CD34, and S-100. In this case, the immunohistochemical staining results were as follows: vimentin (+++), pan-CK (+), desmin (-), c-kit (+), S-100 (-), CD30 (-), and DOG-1 (-). These immunohistochemical markers were suggestive of SCA.
There is no official treatment guideline for SCA, but wide excision including the tumor is the main goal of treatment. There are cases in which adjuvant chemotherapy using 5-FU and/or cisplatin and radiotherapy were administered, but no report identified improvements in survival. However, because of the small number of cases, it is impossible to reach an accurate conclusion concerning the efficacy of those treatments.14
The prognosis of small intestinal carcinoma is typically poor, but SCA appears to have a far worse prognosis. The tumor itself has a high level of invasiveness, and at the time of diagnosis, it is already locally advanced or has metastasized to the liver and lymph nodes. Even with surgery, the expected survival time is usually a few months, and it is extremely rare for a patient to survive for more than 5 years.110 The patient in this report also had adjacent sigmoid colon invasion and multiple liver metastases at the time of diagnosis, and after surgery, he was scheduled to undergo palliative chemotherapy. However, the patient refused to undergo chemotherapy, and he is currently receiving conservative treatment.
In summary, SCA of the small intestine is rare, but it progresses quickly and metastasizes with ease, resulting in a high mortality rate. Surgery is the primary treatment modality, whereas the effects of chemotherapy or radiotherapy have not yet been determined. SCA of the small intestine must be distinguished from other malignant tumors of the small intestine, and if a small intestinal mass is found in middle-aged or elderly patients, there is an immediate need for surgical resection for an accurate diagnosis and treatment. As more cases are gathered, there is a need to research early diagnosis methods such as the use of tumor markers in addition to more effective treatment methods for SCA.
Figures and Tables
References
1. Reid-Nicholson M, Idrees M, Perino G, Hytiroglou P. Sarcomatoid carcinoma of the small intestine: a case report and review of the literature. Arch Pathol Lab Med. 2004; 128:918–921.
2. Moriwaki Y, Sugiyama M. Severe anemia inducing preshock caused by sarcomatoid carcinoma of the small intestine. Int Surg. 2009; 94:164–170.
3. Yucel AF, Kocakusak A, Arikan S, Demirbag N, Tarlaci A, Batur S. A rare cause of acute abdomen: perforated primary sarcomatoid carcinoma of the small intestine: report of a case, with a brief review of the literature. J Cancer Res Ther. 2011; 7:348–350.
4. Conzo G, Giordano A, Candela G, Insabato L, Santini L. Colonic carcinosarcoma. J Gastroenterol Hepatol. 2003; 18:748–749.
5. Robey-Cafferty SS, Silva EG, Cleary KR. Anaplastic and sarcomatoid carcinoma of the small intestine: a clinicopathologic study. Hum Pathol. 1989; 20:858–863.
6. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.
7. Iezzoni JC, Mills SE. Sarcomatoid carcinomas (carcinosarcomas) of the gastrointestinal tract: a review. Semin Diagn Pathol. 1993; 10:176–187.
8. Dikman SH, Toker C. Enteroblastoma complicating regional enteritis. Gastroenterology. 1973; 65:462–466.
9. Radi MF, Gray GF Jr, Scott HW Jr. Carcinosarcoma of ileum in regional enteritis. Hum Pathol. 1984; 15:385–387.
10. Lee SE, Park SY. Sarcomatoid carcinoma of the small intestine: a rare and highly aggressive tumor. J Korean Surg Soc. 2012; 83:321–324.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download