Abstract
Prostate cancer is the second most common cause of cancer death in men in the United States. The most common sites of metastasis include the bone, lymph nodes, lung, liver, pleura, and adrenal glands, whereas metastatic prostate cancer involving the gastrointestinal tract has been rarely reported. A 64-year-old African-American man with a history of prostate cancer presented with anemia. He reported the passing of dark colored stools but denied hematemesis or hematochezia. Colonoscopy revealed circumferential nodularity, and histology demonstrated metastatic carcinoma of the prostate. Esophagogastroduodenoscopy showed hypertrophic folds in the gastric fundus, and microscopic examination revealed tumor cells positive for prostate-specific antigen. Bone scanning and computed tomography of the abdomen and pelvis did not show metastasis. It is crucial to distinguish primary gastrointestinal cancer from metastatic lesions, especially in patients with a history of cancer at another site, for appropriate management.
Prostate cancer is the second most common cause of cancer death in men in the United States. In the United States, the age-adjusted number of new cases of prostate cancer was 147.8 per 100,000 men per year based on 2007~2011 data and the age-adjusted number of deaths was 23.0 per 100,000 men per year based on 2006~2010 data. According to 2008~2010 data, approximately 15.3% of men will be diagnosed with prostate cancer at some point during their lifetime, and in 2011, an estimated 2,707,821 men were living with prostate cancer in the United States.1 The most common sites of prostate cancer metastasis include the bone, lymph nodes, lungs, liver, pleura, and adrenal glands.2 Rarely has metastatic prostate cancer been reported to involve the gastrointestinal tract.3,4,5,6,7,8,9,10,11 Here, we report the case of a 64-year-old man with prostate cancer that had metastasized to the rectum and stomach.
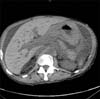
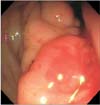
A 64-year-old African-American man presented with anemia (hemoglobin level of 6.8 g/dl) in September 2012. He had a medical history of prostate cancer (Gleason score of 5+4=9) diagnosed in June 2011, hypertension, coronary artery disease with stent placement, and insertion of bilateral percutaneous nephrostomy tubes due to hydronephrosis. The patient was being treated with flutamide and leuprolide acetate for prostate cancer since June 2011. In September 2012, he presented with the complaint of dark-colored stools for a week. At that time, he did not experience any chest pain, shortness of breath, palpitations, dizziness, or headache, and no history of hemoptysis or hematemesis was reported. Physical examination of the patient was unremarkable except for the findings of a cachectic elderly man with pale conjunctiva and bilateral nephrostomy tubes. Laboratory data on admission revealed a hemoglobin level of 6.8 g/dl, hematocrit of 21%, platelet count of 281,000/mm3, white cell count of 4,500/mm3, and normal prothrombin time and activated partial thromboplastin time. Iron studies revealed a total iron level of 80 mg/dl, total iron binding capacity of 227 mg/dl, ferritin level of 357 ng/ml, and reticulocyte count of 0.9%. A complete metabolic profile showed a blood urea nitrogen level of 54 mg/dl and a creatinine level of 3.3 mg/dl. The patient's baseline hemoglobin level at previous admissions was 8 g/dl. He received 2 units of packed red blood cell transfusion in September 2012. The patient had a computed tomography (CT) scan performed without contrast on his abdomen and pelvis. The CT scan revealed multiple cystic lesions in the liver, marked thickening of the wall of the stomach (Fig. 1), and diffuse thickening of the wall of the rectum. The patient also underwent a colonoscopy, which revealed circumferential nodularity and poor distensibility of the rectal lumen (Fig. 2). Biopsy of the rectum indicated diffuse infiltration of high-grade neoplastic cells that were positive for human prostatic acid phosphatase and prostate-specific membrane antigen. These findings were consistent with a diagnosis of metastatic, poorly differentiated carcinoma of the prostate.
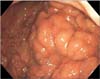
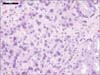
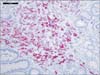
Since it was known that the patient had hormone-refractory prostate cancer, he was started on docetaxel and received 3 cycles until February 2013, at which point he was lost to follow-up. In May 2013, he was admitted because of worsening of his anemia, and esophagogastroduodenoscopy was performed. This revealed hypertrophic folds in the gastric fundus (Fig. 3). Microscopic examination revealed sheets of atypical cells with prominent nucleoli and no glandular pattern within the gastric lamina propria (Fig. 4). Immunohistochemical studies demonstrated that the tumor cells were positive for prostate specific antigen (PSA) and alpha-methylacyl-coenzyme A racemase (AMACR, P504S) (Fig. 5). The patient's serum PSA level was more than 1,000 mg/dl at that time. A bone marrow biopsy did not reveal any malignant infiltration. The bone scan, as well as the CT scan of the abdomen and pelvis, showed no metastases but showed retroperitoneal lymphadenopathy. At that time, the patient had an Eastern Cooperative Oncology Group performance status of 3. The patient and family refused further chemotherapy for metastatic prostate cancer and opted for palliative care instead. The patient was admitted multiple times for similar complaints and was managed with supportive care. However, he died 12 months after he was diagnosed with metastases.
Prostate cancer is the most common noncutaneous cancer in men in the United States and is among the most commonly diagnosed cancers in many developed countries. Classic risk factors for this cancer include older age, African-American race/ethnicity, and a family history of prostate cancer. Prostate cancer incidence rates vary widely between countries. The increase in prostate cancer incidence rates in groups that have migrated from countries with low rates to countries with high rates strongly suggests the importance of environmental factors in its etiology. Kolonel12 reported that the the incidence rates of prostate cancer steadily increased in the Japanese group with migration from mainland Japan to Hawaii, and the United States mainland in that order based on the ethnic studies. The majority of men are diagnosed with prostate cancer at an age older than 65 years, and the vast majority of prostate cancer deaths occur in this older age group. The median age at prostate cancer diagnosis is 71 years in Caucasians and 69 years in African-Americans in the United States.
Metastatic prostate cancer has a poor prognosis and median survival time ranges from 1 to 3 years.3 Prostate cancer preferentially spreads to the skeleton. More than 80% of men who die from prostate cancer are identified with bone metastases at autopsy.13 In contrast to most other cancers, prostate cancer predominantly forms osteoblastic metastases. The vertebral column, pelvis, ribs, and proximal long bones are the most common sites of skeletal metastases. Hematogenous, lymphatic, and direct infiltrations are the typical routes of spread.14 Patients with prostate cancer can be anemic due to bone marrow involvement. Metastasis of prostatic carcinoma to the gastrointestinal tract is a very rare occurrence and presents a diagnostic challenge.6,7,8,9,10,11,13,14 Because the prostate is richly supplied with lymphatic channels, metastasis to the gastrointestinal tract may occur via the lymphatic route. This should be taken into consideration during the work up for anemia in a patient with advanced prostate cancer, as supportive management will depend on the etiology of the anemia. In patients with suspected gastrointestinal bleeding or signs of iron deficiency anemia, upper and lower endoscopy should be considered. Two postmortem studies reported gastric metastasis from primary prostate cancer in 1% to 4% of cases.15,16
Histologically, primary adenocarcinoma of the stomach is composed of atypical glands with cribriform (back to back) formation and a mucin-producing infiltrating growth pattern. Carcinoma in situ or high-grade dysplasia is usually found adjacent to the carcinoma. In contrast, tumor cells of metastatic carcinoma from prostate cancer usually have prominent nucleoli and no glandular formation. The prostate biomarker AMACR has been used in conjunction with morphology with very high sensitivity and specificity in diagnostically challenging cases. This has increased the diagnostic accuracy of prostate cancer worldwide. AMACR, also known as racemase or P504S, is an enzyme identified by cDNA subtraction and microarray technology. It is a sensitive and specific immunohistochemical marker that has been found to be consistently up-regulated in prostate carcinoma.17,18
Treatment for metastatic prostate cancer is palliative. Androgen deprivation therapy is the mainstay of therapy for metastatic prostate cancer. Several new agents have been introduced for the treatment of metastatic prostate cancer in the past two decades, with excellent disease control and good patient tolerability. The management of advanced prostate cancer with metastases to the gastrointestinal tract includes local control measures, supportive care, and treatment of the underlying cancer. If the disease progresses and hormone-refractory metastatic prostate cancer is diagnosed, alternative treatments include chemotherapy, immunotherapy with sipuleucel-T, androgen receptor antagonist drugs such as enzalutamide, and androgen synthesis inhibitors such as abiraterone.
Although rare, it is important to consider the possibility of prostate carcinoma metastasizing to the gastrointestinal tract in patients presenting with gastrointestinal bleeding and a history of prostatic adenocarcinoma. It is crucial to distinguish primary gastrointestinal cancer from metastatic lesions, especially in cases of a previous history of cancer at another site, for appropriate management. This can be achieved by determining the histopathologic classification of the tumor and by immunohistochemical staining for PSA.
Figures and Tables
Fig. 1
Computed Tomography of abdomen without contrast showing marked thickening of the wall of the stomach.
References
1. National Cancer Institute (NCI). SEER cancer status review 1975-2011 [Internet]. USA: NCI;cited 2014 Jun 15. Available from: http://seer.cancer.gov/csr/1975_2011/results_merged/sect_23_prostate.pdf.
2. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000; 31:578–583.

3. Christoph F, Grünbaum M, Wolkers F, Müller M, Miller K. Prostate cancer metastatic to the stomach. Urology. 2004; 63:778–779.

4. Ben-Izhak O, Lichtig C. Signet-ring cell carcinoma of the prostate mimicking primary gastric carcinoma. J Clin Pathol. 1992; 45:452–454.

5. Holderman WH, Jacques JM, Blackstone MO, Brasitus TA. Prostate cancer metastatic to the stomach. Clinical aspects and endoscopic diagnosis. J Clin Gastroenterol. 1992; 14:251–254.

6. Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990; 65:1596–1600.

7. Nakamura T, Mohri H, Shimazaki M, Ito Y, Ohnishi T, Nishino Y, et al. Esophageal metastasis from prostate cancer: diagnostic use of reverse transcriptase-polymerase chain reaction for prostate-specific antigen. J Gastroenterol. 1997; 32:236–240.

8. Gore RM, Sparberg M. Metastatic carcinoma of the prostate to the esophagus. Am J Gastroenterol. 1982; 77:358–359.
9. Eaves R, Lambert J, Rees J, King RW. Achalasia secondary to carcinoma of prostate. Dig Dis Sci. 1983; 28:278–284.

10. Malhi-Chowla N, Wolfsen HC, Menke D, Woodward TA. Prostate cancer metastasizing to the small bowel. J Clin Gastroenterol. 2001; 32:439–440.

11. Onitilo AA, Engel JM, Resnick JM. Prostate carcinoma metastatic to the stomach: report of two cases and review of the literature. Clin Med Res. 2010; 8:18–21.

12. Kolonel LN. Cancer patterns of four ethnic groups in Hawaii. J Natl Cancer Inst. 1980; 65:1127–1139.
13. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000; 31:578–583.

15. Oda I, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001; 33:507–510.

16. Mintz ER, Smith GG. Autopsy findings in 100 cases of prostate carcinoma. N Eng J Med. 1934; 211:479–487.
17. Jiang Z, Wu CL, Woda BA, Dresser K, Xu J, Fanger GR, et al. P504S/alpha-methylacyl-CoA racemase: a useful marker for diagnosis of small foci of prostatic carcinoma on needle biopsy. Am J Surg Pathol. 2002; 26:1169–1174.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download