Abstract
Purpose
Gastric cancer often occurs in the elderly but is uncommon in young individuals. Whether young patients have different clinical behaviors and outcomes from those of older patients remain unclear.
Materials and Methods
We identified 1,366 cases of newly diagnosed noncardia gastric adenocarcinoma from the Kaiser Permanente Northern California Cancer Registry between 2000 and 2010. We then compared the clinicopathological features and survival among the different age groups.
Results
The male : female ratio differed significantly between the younger and older patient groups (0.84 in age <50 years vs. 1.52>60 years, P<0.01). More younger patients were Hispanic (54% patients <40 years vs. 19% patients ≥70 years, P<0.0001), while more older patients were Caucasian (49% patients ≥70 years vs. 15% patients <40 years; P<0.0001). The diffuse/mixed histological type was more prevalent in younger patients (70% patients <40 years vs. 27% patients ≥70 years; P<0.0001), whereas the intestinal type was more frequent in older patients (71% in patients ≥70 years vs. 30% in patients <40 years; P<0.0001). Poorly differentiated adenocarcinoma was more common in the younger patients (80% in patients <40 years vs. 60% in patients ≥70 years; P=0.016). Survival rates at 1, 2, and 5 years gradually declined with increasing age (overall P=0.0002).
Conclusions
Young patients with gastric cancer had more aggressive disease but higher overall survival rates than older patients. Younger Hispanic patients and older Caucasian patients were more likely to be diagnosed with gastric cancer. These differences may be due to biological predisposition and/or environmental exposure.
Gastric cancer remains the second most common cause of cancer-related death worldwide. In the United States, approximately 21,600 patients are diagnosed and 10,990 patients die annually.1 The development of gastric cancer is thought to result from a combination of genetic predisposition, environmental exposure, and socioeconomic factors.2
Gastric cancer is most commonly diagnosed in individuals 65 to 74 years of age.3 However, a small proportion of patients are diagnosed at <40 years of age. Studies have suggested that younger patients may have distinct disease characteristics.4,5 In particular, younger patients may be more likely to be female5,6 and have a higher incidence of diffuse histologic type, advanced grade, and metastatic disease at diagnosis.7,8,9 Younger patients have a higher prevalence of a positive family history.10
Most studies investigating the impact of age on gastric cancer have been conducted internationally, particularly in Asian countries, while only a few studies have been performed in the USA.7,8,9,10,11 These studies have several limitations, including the inability to access patient-level data in those studies using large national registries, heterogeneous study populations, and varying access to care that may have a substantial impact on survival. In addition, most studies did not differentiate between cardia and noncardia gastric adenocarcinoma, which are known to be biologically distinct diseases.12 The age cutoff for defining young or old patients has differed significantly among studies.6,7,9 Furthermore, findings comparing prognosis between younger and older patients have been inconsistent.13,14 Some studies showed that young patients had worse survival rates due to more aggressive disease at presentation and delayed diagnosis.8,15 Other studies demonstrated that, when matched for stage, younger patients did not have worse outcomes.7,16
Overall, whether young patients with gastric cancer have distinct disease patterns and differences in survival rates remains poorly understood. In this study, we investigated the impact of age on the clinicopathological features and survival of patients with gastric cancer in an integrated health care delivery system in the USA. We hope to better define age-specific patterns of gastric cancer and possible associated risk factors. This in turn will help health care providers personalize care for patients with gastric cancer in each age group to improve survival rates.
This was a retrospective cohort study of patients from the Kaiser Permanente Northern California (KPNC) cancer registry who were newly diagnosed with noncardia gastric adenocarcinoma between 2000 and 2010. KPNC is a large integrated health care delivery system that provides care for approximately 3.2 million members annually in Northern California, comprising one third of insured adults in the area. The population is racially and ethnically diverse, which closely approximates the general population in Northern California.17,18
Subjects were identified using International Classification of Diseases diagnostic codes and Surveillance, Epidemiology, and End Results (SEER) site recodes from the KPNC cancer registry. Study subjects were required to have a KPNC membership for a minimum of 12 months prior to the diagnosis of gastric cancer to ensure the availability of adequate baseline data.
Demographics, including age at diagnosis, gender, race/ethnicity (non-Hispanic Caucasian, Asian, African-origin, and Hispanic) and tumor-related information, were extracted from the KPNC cancer registry or obtained and validated by individual medical record review (M.C.B. and D.L). All cases of noncardia gastric adenocarcinoma were pathologically confirmed. Cases of gastric cancer of the cardia (C160) were excluded. Disease information, including primary tumor location, grade, and stage, were obtained. The primary tumor location was further classified as follows: fundus (C161), body (C162, C165, and C166), antrum/pylorus (C163 and C164), overlapping lesions (C168), and not specified (C169). Grade was defined as well differentiated, moderately differentiated, poorly differentiated/undifferentiated, and unknown. Stage at presentation was categorized as in situ/localized, regional, metastatic, or unknown. Histological type was categorized based on Lauren classification as intestinal, diffuse/mixed, or indeterminate.19
Treatment modalities, including surgery, chemotherapy, and radiation, were obtained. Only surgeries with curative intent, such as partial or total gastrectomy and polypectomy, were included. Mortality data was obtained from the California state mortality files and virtual data warehouse mortality files. Survival time was measured in days from the date of diagnosis of gastric cancer to the date of death. For non-deaths, overall survival was censored at December 31, 2011.
Major comorbidities including coronary artery disease, hypertension, chronic obstructive pulmonary disease, and diabetes mellitus were included as confounding factors. History of Helicobacter pylori infection was confirmed by serology and from pathology reports.
Subjects were divided into five age groups as follows: <40 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, and ≥70 years. Demographic (gender and race/ethnicity), clinicopathological features, and survival were compared among the different age groups. The annual incidence rates of noncardia gastric cancer per 100,000 persons between 2000 and 2010, according to age group were then calculated.
Demographic and clinicopathological features were compared between different age groups using the chi-square or Fisher exact test for categorical variables as appropriate. MULTTEST was used to compare the age groups in pairs and control for multiple comparisons. The annual incidence rates of noncardia gastric adenocarcinoma were calculated by age group between 2000 and 2010. The Cochran-Armitage trend test was performed to demonstrate changes in the incidence rates of noncardia gastric adenocarcinoma over time according to age.
Kaplan-Meier analysis was used to compare 1-, 2-, and 5-year survival rates among the different age groups. The log-rank test was used to assess differences in survival rates. Statistical analysis was performed using SAS (SAS Institute, Cary, NC, USA). P-values <0.05 were considered statistically significant.
This study was approved by the Kaiser Foundation Research Institute's institutional review board.
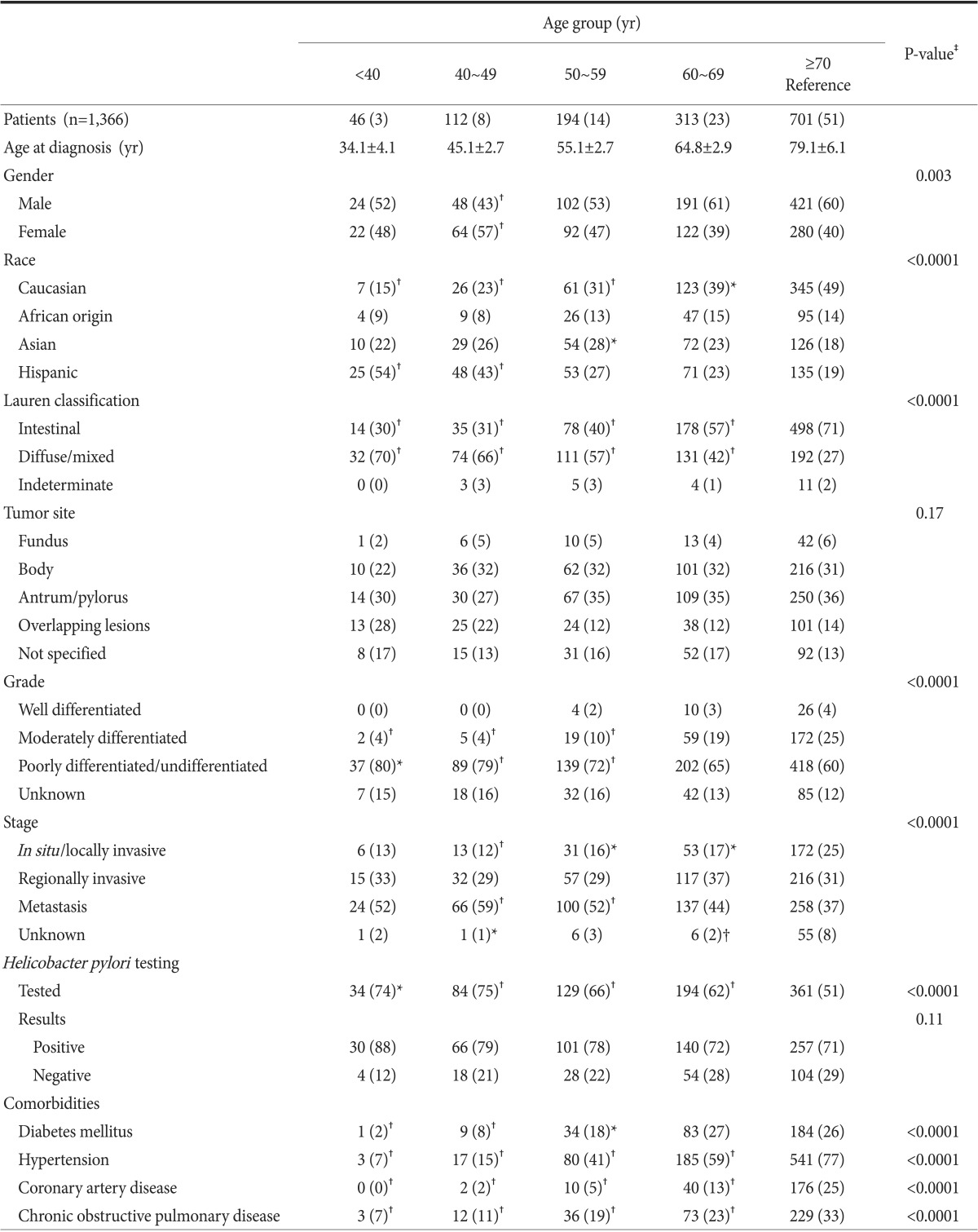
Between 2000 and 2010, a total of 1,378 cases of newly diagnosed noncardia gastric adenocarcinoma were identified. Twelve cases were excluded owing to missing information, resulting in a study population of 1,366. Demographic and clinicopathological features of the study population are compared according to age, as presented in Table 1. Subjects were 25 to 101 years old. For all individuals, the mean and median ages at the time of diagnosis were 68 and 70 years, respectively. The age distribution of the study population is shown in Table 1.
In terms of gender, there was a significantly higher percentage of females only in the 40 to 49 year age group compared to patients ≥70 years (57% vs. 40%, P=0.003). There was a significantly higher proportion of Hispanics among the younger subjects (54% in patients <40 years vs. 19% in patients ≥70 years, P<0.0001). In contrast, there were more Caucasians in the older groups (49% in patients ≥70 years vs. 15% in patients <40 years; P<0.0001) (Table 1).
With regard to Lauren classification, the diffuse/mixed histological type was more prevalent in younger subjects (70% of patients <40 years and 66% of patients 40~49 years vs. 42% of patients 60~69 years and 27% of patients ≥70 years; P<0.0001). In contrast, the intestinal type was more frequent in the older groups (71% of patients ≥70 years and 57% of patients 60~69 years vs. 31% of patients 40~49 years and 30% of patients <40 years; P<0.0001). There were no statistically significant differences in tumor location. Moderately differentiated adenocarcinoma was more prevalent in older subjects (25% of patients ≥70 years vs. 4% of patients <40 years, P=0.003). In contrast, poorly differentiated adenocarcinoma was more common in the younger subjects (80% of patients <40 years vs. 60% of patients ≥70 years, P=0.02). In addition, there was a higher prevalence of metastatic disease in the younger subjects (37% of patients ≥70 years vs. 59% of patients 40~49 years; P<0.0001).
Although a higher proportion of younger subjects were tested for H. pylori infection (P<0.0001), the percentages of H. pylori infection did not differ statistically among the age groups. With regard to major co-morbidities, the prevalence of diabetes, hypertension, coronary artery disease, and chronic obstructive pulmonary disorder was significantly higher in the older age groups.
The annual incidence of noncardia gastric adenocarcinoma by age group from 2000 to 2010 is shown in Fig. 1. The incidence rates were significantly higher in the older age groups (60~69 years and ≥70 years) than the younger age groups (<60 years). A trend analysis showed that the incidences decreased in the older age groups (60~69 years, P=0.0008; and ≥70 years, P=0.0002) during the study period but remained relatively stable in the younger age groups.
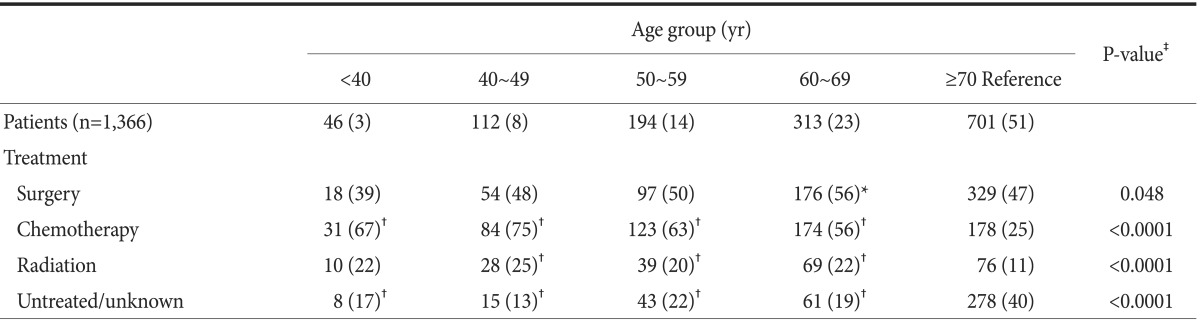
Treatment type according to age group is summarized in Table 2. The rates of surgical intervention were 39% to 56%. The rate of surgery was significantly higher in the 60 to 69 years group than in the ≥70 years group (56% vs. 47%, P=0.026). The percentage of subjects who received chemotherapy was significantly higher in all age groups than that in the ≥70 years group (P<0.0001). The rate of radiation therapy was similar in most age groups but was lower in ≥70 years. The percentage of subjects who did not undergo any treatment or had unknown treatment status was highest in the ≥70 years group.
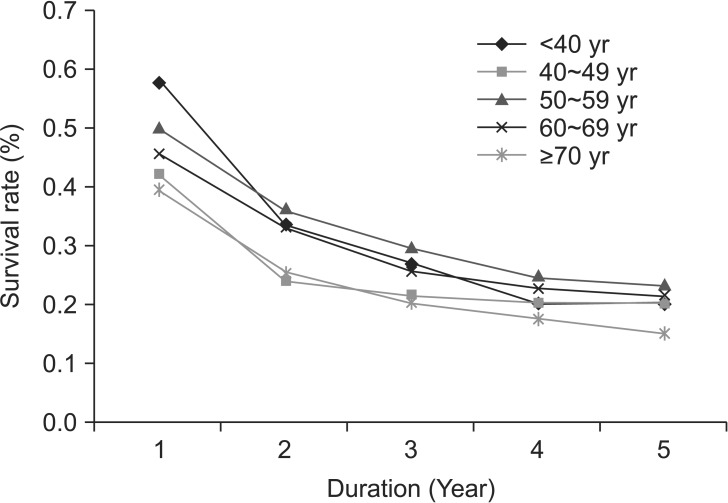
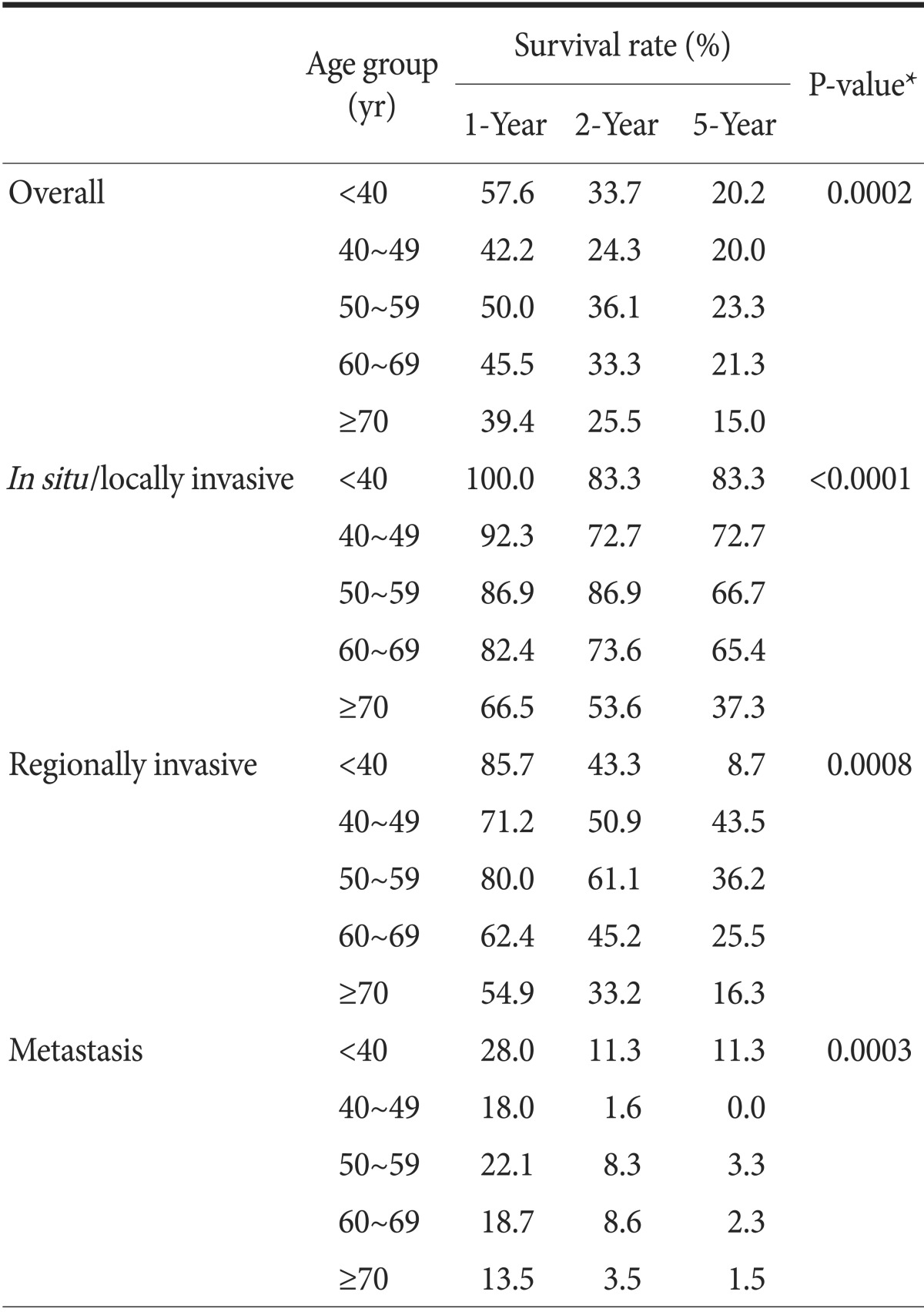
Survival rates over 5 years from all-cause mortality are shown in Fig. 2. Survival rates gradually declined with increasing age (P=0.0002) with the exception of the 40 to 49 years group, which had lower survival. One-year survival rates were 57.6% for the <40 years group vs. 39.4% for the ≥70 years group (Table 3). Five-year survival rates were 20.2% for the <40 years group vs. 15% for the ≥70 years group. Although patients with gastric cancer in the younger age groups had more aggressive disease, their overall survival rates were non-inferior to those of patients ≥70 years.
Table 3 also displays the survival rates stratified by stage, an important prognostic factor. For in situ/locally invasive tumors, survival declined with increasing age. For regionally invasive disease, the survival rates were lower in the <40 years group at 2 and 5 years. For metastatic disease, survival decreased with increasing age with the exception of the 40 to 49 years group, which had the lowest survival.
Within Kaiser Permanente's health care delivery system in Northern California, we identified several differences in the demographic and clinicopathological features of noncardia gastric adenocarcinoma between young and old subjects. Consistent with previous studies, we found that younger subjects with gastric cancer more commonly had diffuse histological type, advanced grade, and metastatic disease at the time of diagnosis compared with older subjects.4,9 Although subjects <60 years had more aggressive tumor biology on presentation, overall younger age was not associated with worse survival with the exception of the 40 to 49 years group, which had the worst survival among the subjects <70 years.
Studies have reported conflicting results regarding survival rates of younger patients with gastric cancer. A recent Japanese study found that patients ≤40 years had significantly lower overall survival than patients >40 years. The poorer survival in the young patient groups was thought to be secondary to aggressive tumor biology and advanced stage. However, among those who underwent curative resection, patients ≤40 years had similar 5-year survival rates than those >40 years.6 Similarly, a USA study at Harbor-The University of California, Los Angeles Medical Center found that patients ≤35 years had lower curative surgery rates and worse postoperative outcomes compared with patients >35 years.8 The worse survival rates in younger patients were attributed to their more aggressive tumor biology. In contrast, a large population-based study from the USA using the SEER registry showed that although a higher proportion of young patients had more advanced stage and higher histological grades, the 5-year survival rates stratified by stage were better in young patients than in older patients.7
Taken together, these studies suggested that multiple age-related factors can influence gastric cancer survival. Young patients tend to have more aggressive tumor biology, likely due to genetic predisposition, which at least partially contributes to their more advanced stage at the time of diagnosis compared with older patients. Secondly, clinical suspicion for malignancy in younger patients is generally lower than in older patients, which may lead to a significantly delay in diagnosis and treatment of gastric cancer until it reaches a more advanced stage. On the other hand, young patients are generally healthier and have fewer comorbidities than older patients, because of which they can receive more aggressive treatment, such as surgery, chemotherapy, and radiation therapy.14,20 Our findings that young patients, despite having more aggressive histology and advanced stage, had overall improved survival rates than older patients support this assumption.
The incidences decreased in the older age groups during the study period but remained relatively stable in the younger groups. Recent epidemiological studies using the USA National Cancer Institute SEER registry showed that the incidence of noncardia gastric adenocarcinoma decreased in all of the race groups except for Caucasians 25 to 39 years, among whom it increased.11 Reasons for this discrepant trend remain unknown. One speculative explanation was that the eradication of H. pylori may have unmasked other possible carcinogenic processes. For example, a change in gastric microbial flora may increase the exposure to other carcinogenic agents such as the Epstein-Barr virus, and the effect may be more profound in younger patients.21,22 The widespread use of acid inhibitors may also play a role in initiating precancerous changes in gastric mucosa, although additional investigations are needed to verify this hypothesis.11
The distinctive age-related features of gastric cancer likely reflect the underlying differences in the pathogenesis and molecular biology of gastric cancer.23,24,25 In young patients, the development of gastric cancer is more likely influenced by genetic predisposition. Not surprisingly, a high prevalence of positive family history has been observed in other studies among patients who were diagnosed with gastric cancer at a younger age.10 Therefore, clinicians should be more vigilant when a positive family history of gastric cancer exists in a young patient who presents with suspicious symptoms and signs, particularly in Hispanics (see discussion below).15 In contrast, gastric cancer in older individuals may be more strongly associated with long-term exposure to environmental factors such as H. pylori infection or dietary exposure to carcinogens. For example, the intestinal histological type is more common in elderly subjects, which is known to develop through a cascade of events from chronic gastritis to intestinal metaplasia to adenocarcinoma.
Similar to other studies, we noted a higher female predominance among younger subjects and a lower prevalence among older subjects. Reasons for this discrepancy remain unknown but hormonal differences may play a role. Interestingly, a recent meta-analysis26 found that longer years of fertility and hormone replacement therapy were associated with a decreased cancer risk, suggesting that longer exposure to estrogen with increasing age may actually be protective.
We found that Hispanics were significantly younger at diagnosis compared with other racial groups. This difference has also been observed in other USA studies.20,27,28,29 A study using the National Cancer Database found that Hispanics were more likely to be younger (<50 years) at diagnosis than Caucasians and African Americans.27 Similarly, a study examining survival rates of metastatic gastric cancer using SEER registries found that 19% of Hispanic patients were diagnosed at <45 years compared to 5.5% of Caucasians.20 At the regional level, a study using patients from the Los Angeles County Cancer Surveillance Program also found that Hispanics were youngest at diagnosis and Caucasians were oldest at diagnosis.28 Another retrospective study of patients treated over a 15-year period at a single comprehensive cancer center in Texas found that Hispanics were significantly younger at diagnosis than non-Hispanics.29 It is likely that the higher incidence of H. pylori infection led to a higher rate of gastric cancer in young Hispanic subjects. However, H. pylori infection is unlikely to be the sole cause, since African-Americans also have a higher prevalence of H. pylori infection but a later onset of gastric cancer.29,30 It remains possible that a difference in genetic predisposition exists between Hispanics and other racial groups. Other environmental factors, particularly dietary habits, may also be responsible and warrant further investigation.
Our study had several strengths. In contrast to large population-based studies, we were able to obtain most variables from electronic medical records, databases, and registries, and when necessary perform individual chart reviews to obtain or validate subject-level data such as histological type, history of H. pylori infection, treatment received, and comorbidities. In addition, all of our patients were insured and had relatively equal access to care, which allowed us to focus on specific variables that influence disease behaviors and outcomes in gastric cancer.
Our study had a few limitations. First, it did not include the uninsured population or patients who received health care in a fee-for-service or non-integrated setting. The outcomes of patients with gastric cancer in these health care settings are likely different, particularly due to variability in access to care. Second, we were unable to obtain information on variables such as family history, smoking history, and environmental factors such as migration patterns or dietary habits for all study subjects, which may be important predictors for gastric cancer. Third, compared with those in large registry-based studies, our study population was small, which limits our capacity to detect more age-related differences in gastric cancer.
In conclusion, here we identified significant age-specific differences in the demographic features, clinicopathological features, and survival rates among subjects with noncardia gastric adenocarcinoma. We found that young subjects were more likely to have aggressive disease, although their overall survival rates were non-inferior compared with subjects ≥70 years. Age at diagnosis was significantly younger in Hispanics compared with other racial groups. Since gastric cancer survival remains poor, further investigations of the genetic, molecular, and environmental factors underlying these age-related differences will provide important new insights and ultimately improve patient survival.
Acknowledgments
An abstract of this study was presented at Digestive Disease Week in Orlando, Florida in May 2013.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30. PMID: 23335087.

2. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013; 42:211–217. PMID: 23639637.
3. Surveillance, Epidemiology, and End Results (SEER). SEER Stat Fact Sheets: Stomach Cancer [Internet]. Bethesda (MD): SEER;cited 2014 Apr 6. Available from: http://seer.cancer.gov/statfacts/html/stomach.html.
4. Kong X, Wang JL, Chen HM, Fang JY. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol. 2012; 106:346–352. PMID: 22331676.

5. Saito H, Takaya S, Fukumoto Y, Osaki T, Tatebe S, Ikeguchi M. Clinicopathologic characteristics and prognosis of gastric cancer in young patients. Yonago Acta Med. 2012; 55:57–61. PMID: 24031140.
6. Isobe T, Hashimoto K, Kizaki J, Miyagi M, Aoyagi K, Koufuji K, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013; 30:43–49. PMID: 23674196.

7. Al-Refaie WB, Hu CY, Pisters PW, Chang GJ. Gastric adenocarcinoma in young patients: a population-based appraisal. Ann Surg Oncol. 2011; 18:2800–2807. PMID: 21424881.

8. Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg. 2009; 144:506–510. PMID: 19528381.

9. Theuer CP, Kurosaki T, Taylor TH, Anton-Culver H. Unique features of gastric carcinoma in the young: a population-based analysis. Cancer. 1998; 83:25–33. PMID: 9655289.
10. Koea JB, Karpeh MS, Brennan MF. Gastric cancer in young patients: demographic, clinicopathological, and prognostic factors in 92 patients. Ann Surg Oncol. 2000; 7:346–351. PMID: 10864341.

11. Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010; 303:1723–1728. PMID: 20442388.

12. Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol. 2011; 106:1978–1985. PMID: 22008896.

13. Park JC, Lee YC, Kim JH, Kim YJ, Lee SK, Hyung WJ, et al. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol. 2009; 99:395–401. PMID: 19347884.

14. Ramos-De la, Salgado-Nesme N, Torres-Villalobos G, Medina-Franco H. Clinicopathologic characteristics of gastric cancer in a young patient population. J Gastrointest Surg. 2004; 8:240–244. PMID: 15019915.
15. López-Basave HN, Morales-Vásquez F, Ruiz-Molina JM, Namendys-Silva SA, Vela-Sarmiento I, Ruan JM, et al. Gastric cancer in young people under 30 years of age: worse prognosis, or delay in diagnosis? Cancer Manag Res. 2013; 5:31–36. PMID: 23580357.

16. Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007; 94:737–742. PMID: 17330827.

17. Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013; 11:172–180. PMID: 22985608.

18. Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. Internal Division of Research Report. Oakland (CA): Kaiser Permanente Division of Research;2012. cited 2013 Nov. Available from: http://www.dor.kaiser.org/external/DORExternal/mhs/special_reports.aspx.
19. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49. PMID: 14320675.
20. Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, et al. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011; 2:77–84. PMID: 22811834.
21. Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009; 7:887–894. PMID: 19898491.

22. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009; 137:824–833. PMID: 19445939.

23. Buffart TE, Carvalho B, Hopmans E, Brehm V, Kranenbarg EK, Schaaij-Visser TB, et al. Gastric cancers in young and elderly patients show different genomic profiles. J Pathol. 2007; 211:45–51. PMID: 17117405.

24. Carvalho R, Milne AN, van Rees BP, Caspers E, Cirnes L, Figueiredo C, et al. Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas occurring at a later age. J Pathol. 2004; 204:75–83. PMID: 15307140.

25. Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013; 145:554–565. PMID: 23684942.

26. Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012; 21:20–38. PMID: 22028402.

27. Al-Refaie WB, Tseng JF, Gay G, Patel-Parekh L, Mansfield PF, Pisters PW, et al. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer. 2008; 113:461–469. PMID: 18553367.
28. Kim J, Sun CL, Mailey B, Prendergast C, Artinyan A, Bhatia S, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol. 2010; 21:152–160. PMID: 19622590.

29. Yao JC, Tseng JF, Worah S, Hess KR, Mansfield PF, Crane CH, et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution's experience over 15 years. J Clin Oncol. 2005; 23:3094–3103. PMID: 15860869.

30. Yao JC, Schnirer II, Reddy S, Chiang S, Najam A, Yu C, et al. Effects of sex and racial/ethnic group on the pattern of gastric cancer localization. Gastric Cancer. 2002; 5:208–212. PMID: 12491078.

Fig. 1
Annual incidence of noncardia gastric adenocarcinoma between 2000 and 2010 at Kaiser Permanente Northern California, according to age group. Trend analysis: <40, P=0.62; 40~49, P=0.49; 50~59, P=0.71; 60~69, P=0.0008; ≥70, P=0.0002.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download