Abstract
Purpose
This study aimed to evaluate the value of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels to detect gastric cancer recurrence.
Materials and Methods
We retrospectively reviewed 154 patients who developed recurrence within 2 years after curative gastric cancer surgery and analyzed the relationship between postoperative CEA and CA19-9 levels and recurrence. We readjusted the cut-off values to improve the detection of recurrence. Subgroup analysis according to clinicopathologic variables was performed to further investigate the relationship between recurrence and CEA and CA19-9 levels.
Results
The sensitivity and specificity for elevated CEA levels to detect recurrence were 40.6% and 89.5%, respectively, and those for CA19-9 were 34.2% and 93.6%, respectively. The sensitivity and specificity for elevation of either tumor marker were 54.3% and 84.0%, respectively; those for elevation of both tumor markers were 19.2% and 98.4%, respectively. By readjusting the cut-off values from 5.0 ng/ml to 5.2 ng/ml for CEA and from 37.00 U/ml to 30.0 U/ml for CA19-9, the sensitivity was increased from 34.2% to 40.2% for CA19-9, while there was no increase in sensitivity for CEA. In subgroup analysis, the sensitivity of CEA was higher in patients with elevated preoperative CEA levels than in patients with normal preoperative CEA levels (86.7% versus 33.7%; P<0.001). Furthermore, the sensitivity of CA19-9 was higher in patients with elevated preoperative CA19-9 levels than in patients with normal preoperative CA19-9 levels (82.61% versus 26.83%; P<0.001).
Numerous gastric cancer tumor markers related to staging, diagnosis, assessing treatment response, and determining patient prognosis have been used clinically.1,2,3,4,5,6,7,8,9,10,11,12 In addition to traditional tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and CA72-4, there are tumor markers specific to gastric cancer, including alpha-fetoprotein, CA-125, CA-195, CA-50, pepsinogen I/II, soluble E-cadherin, and REG4. However, stomach cancer tumor markers are not sufficient in terms of sensitivity and specificity5 and are rarely implemented when assessing diagnosis and treatment.
After radical gastric cancer surgery, computed tomography (CT), esophagogastroduodenoscopy (EGD), and ultrasonography are the primary methods used to evaluate recurrence.13 However, these methods are relatively expensive and involve potentially hazardous radiation, particularly in the case of CT. Therefore, there are certain limitations to using these methods to routinely assess disease recurrence.
There have been few prior studies evaluating the relationship between postoperative serum tumor marker levels and recurrence after radical gastric cancer surgery;6,11,12,14 conversely, most of these reports demonstrated the relationship between preoperative serum tumor marker levels and diagnosis or prognosis of gastric cancer.1,2,3,4,5,6,7,8,9,10
The aims of this study were to evaluate the association between gastric cancer recurrence and postoperative serum tumor marker levels and to assess the suitability of current serum CEA and CA19-9 cut-off values for the early detection of gastric cancer recurrence. In addition, we performed a subgroup analysis according to clinicopathologic variables to further investigate the relationship between recurrence and levels of CEA and CA19-9.
This retrospective study included 1,515 patients who underwent curative (R0) gastric cancer surgery from January 1, 2005 to December 31, 2006 at Seoul National University Hospital. Patients who underwent gastric cancer surgery for recurrence or metastasis were excluded. Of the 1,515 patients, 154 had confirmed disease recurrence during the 2-year observation period.
Patient follow-up included measurement of serum CEA and CA19-9 levels, along with physical examination, abdominopelvic CT or abdominal sonography, and gastrofiberoscopy, conducted every 6 months. Because disease recurrence in most cases occurs within the first 2 years after surgery, the follow-up period for this study was 2 years.15,16 During that period, 201 patients were lost to the follow-up. The remaining 1,314 patients were included in the final analyses. Clinicopathologic and surgical characteristics, including sex, age, operation type, histological type, stage, recurrence time, recurrence sites, and preoperative tumor marker levels, were obtained through review of medical records. Gastric cancer staging was based on the American Joint Committee on Cancer classification guidelines (7th edition).
Serum levels of CEA and CA19-9 were measured using the immunoradiometric method (the 'sandwich' method) with iodine-125. Cut-off values were 5.0 ng/ml for CEA and 37 U/ml for CA19-9.
In patients with recurrence, confirmed by imaging or pathologic findings, during the follow-up period, postoperative tumor marker levels measured <3 months before or after the time of recurrence were considered. For those without recurrence, the postoperative tumor marker levels considered were the highest levels measured during the follow-up period.
The sensitivity, specificity, positive predictive value, and negative predictive value of each tumor marker to detect recurrence were analyzed using the chi-square test. In addition, we also analyzed the predictive value of the combination of tumor markers.
In order to increase the utility value of serum CEA and CA19-9 levels for the early detection of recurrence after radical gastric cancer surgery, we readjusted the cut-off values of each tumor maker using receiver operating characteristic (ROC) curves and evaluated the clinical utility of these readjusted cut-off values.
For statistical analyses, the statistical software PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA) was used. For the determination of significance, P-values less than 0.05 were considered statistically significant.
This study was approved by Institutional Review Board of Seoul National University Hospital (1404-119-575).
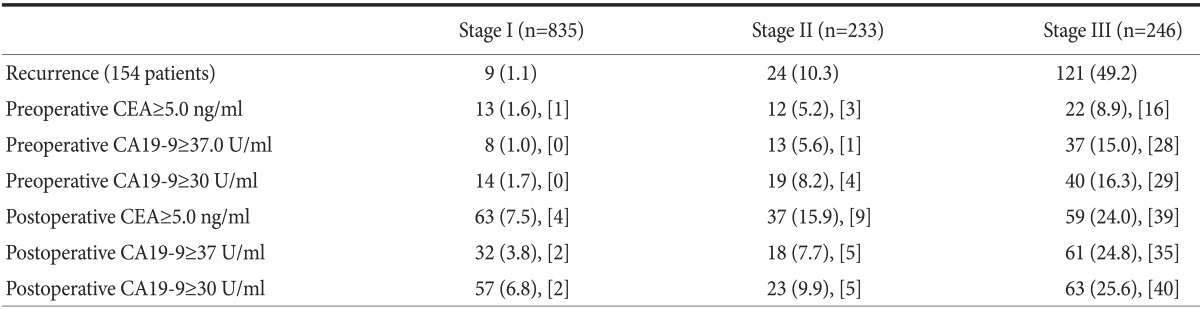
Among 1,314 patients, 154 patients had confirmed disease recurrence during the observation period. Tumor marker levels were measured within 3 months before recurrence in 74.1% of these cases and within 3 months after recurrence in 25.9% of these cases. The number of patients who underwent a partial gastrectomy and total gastrectomy were 1,038 (79.0%) and 276 (21.0%), respectively. There were 835 (63.5%) patients with stage I disease, 233 (16.5%) with stage II disease, and 246 (17.7%) with stage III disease. The mean recurrence time was 10.8 months. The most common recurrence sites were the lymph nodes (n=49; 26.2%), peritoneum (n=45; 24.1%), and liver (n=32; 17.1%). In terms of preoperative tumor maker levels, CEA was elevated in 46 patients (3.5%), CA19-9 was elevated in 55 patients (4.2%), ≥2 tumor markers were elevated in 95 patients (7.2%), and both CEA and CA19-9 were elevated in 6 patients (0.5%) (Table 1).
We then analyzed the relationship between disease recurrence after radical gastric cancer surgery and postoperative serum levels of CEA and CA19-9. With regard to levels of CEA, the sensitivity was 40.6%, the specificity was 89.5%, the positive predictive value was 34.4%, and the negative predictive value was 91.7%. With regard to levels of CA 19-9, the sensitivity was 34.2%, the specificity was 93.6%, the positive predictive value was 41.2%, and the negative predictive value was 91.5%.
For the combination of both CEA and CA19-9, in the case of increased levels of a single marker, the sensitivity was 54.3%, the specificity was 84.0%, the positive predictive value was 32.9%, and the negative predictive value was 92.7%. In the case of increased levels of both markers, the sensitivity was 19.2%, the specificity was 98.4%, the positive predictive value was 60.5%, and the negative predictive value was 90.5% (Table 2).
Our results demonstrated a relationship between recurrence after radical gastric cancer surgery and postoperative serum tumor marker levels when using cut-off values of 5.0 ng/ml for CEA and 37 U/ml for CA19-9, as these cut-off values are commonly utilized in clinical practice.
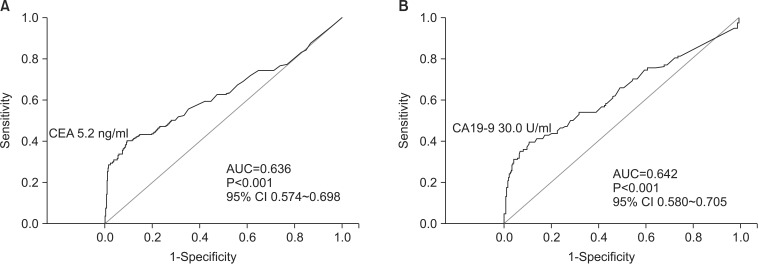
Using ROC curve analysis, we readjusted these cut-off values to 5.2 ng/ml for CEA (Fig. 1A) and to 30.0 U/ml for CA19-9 (Fig. 1B).
We then applied these readjusted cut-off values for early detection of recurrence after radical gastric cancer surgery. In the case of CEA, the sensitivity was 40.6%, the specificity was 90.2%, the positive predictive value was 36.1%, and the negative predictive value was 91.8%. In the case of CA19-9, the sensitivity was 40.2%, the specificity was 89.4%, the positive predictive value was 33.3%, and the negative predictive value was 91.9% (Table 3).
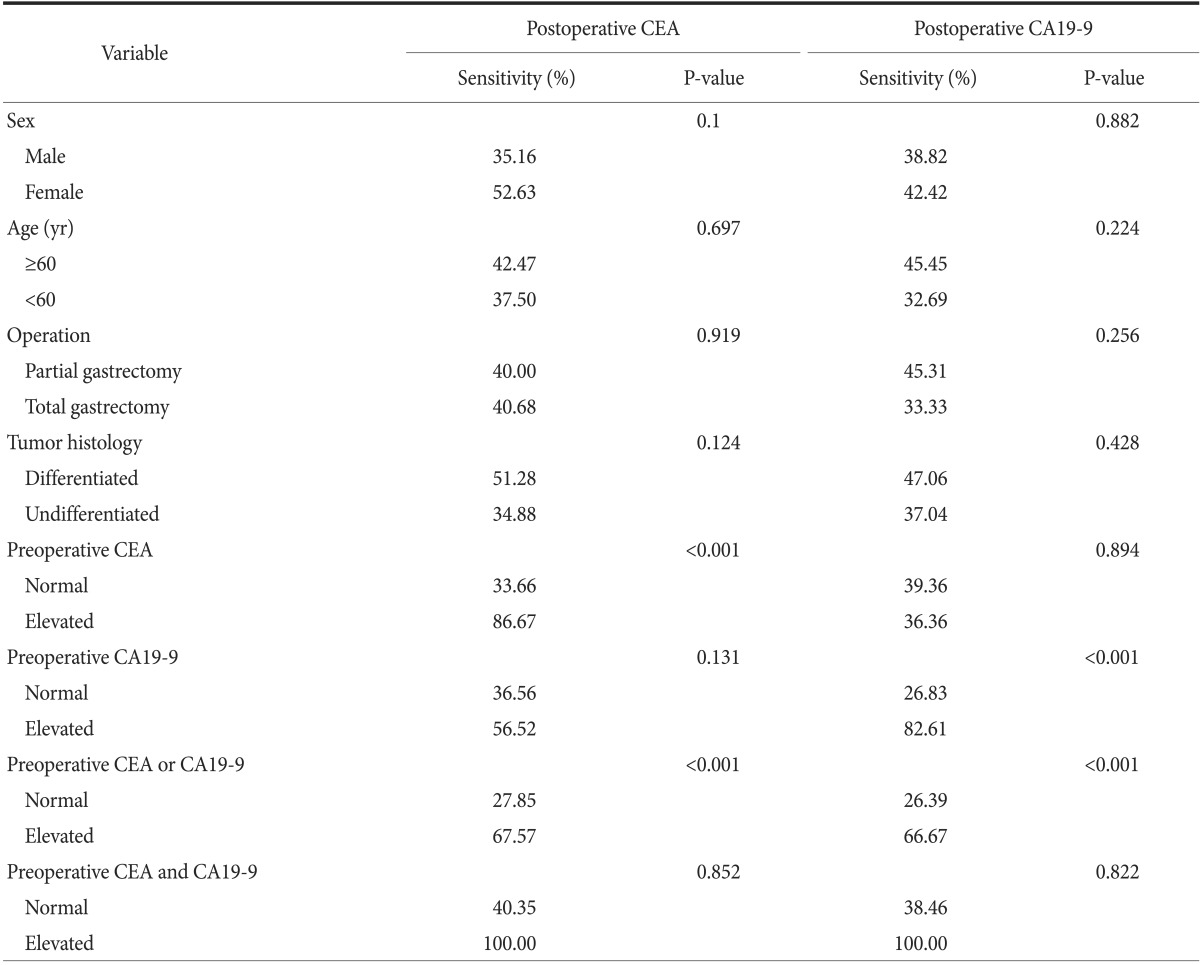
Next, we evaluated whether the sensitivity of postoperative serum CEA and CA19-9 levels for the early detection of recurrence differed according to patients' clinicopathologic and surgical variables. The cut-off values of CEA and CA 19-9 were based on the readjusted values in our study. The results demonstrated that sex, age, operation type, histological type, and stage were not significantly associated with differences in the sensitivity of CEA and CA19-9 levels.
However, the sensitivity of postoperative CEA levels for early detection of recurrence was significantly higher in patients with elevated preoperative CEA levels than in patients with normal preoperative CEA levels (33.66% versus 86.67%; P<0.001). Similarly, the sensitivity of postoperative CA19-9 for the early detection of recurrence was much higher in patients with elevated preoperative CA19-9 levels than in patients with normal preoperative CA19-9 levels (26.83% versus 82.61%; P<0.001). Similar trends were observed for the combination of CEA and CA19-9 (Table 4).
Most of the tumor marker elevation and recurrence were observed for Stage III cancer. Of the 154 patients who displayed recurrences within the observation period of 2 years, 9 patients had Stage I, 24 patients Stage II, and 121 patients Stage III disease.
For Stage III disease, the number of patients with postoperative CEA elevation was 59 (24.0%). The number of patients with postoperative CA19-9 elevation above 37 U/ml was 61 (24.8%), and 63 (25.6%) patients showed CA19-9 levels above 30 U/ml (Table 5).
Various methods have been proposed to detect early recurrence of gastric cancer following radical surgery. However, with the exception of CT, EGD, ultrasonography, and measurement of blood tumor markers, few methods have shown promise for clinical use. Moreover, CT, EGD, and ultrasonography are relatively expensive methods and may be too burdensome to patients.
Similar to the majority of tumor markers, there are certain limitations to using CEA and CA19-9 levels to detect recurrence after radical gastric cancer surgery; particularly, these markers have low sensitivity. According to Marrelli et al.,6 the sensitivity in patients with recurrences was 44% for CEA and 56% for CA 19-9, while the specificity was 79% for CEA and 74% for CA 19-9. The positive predictive value was 73% for CEA and 74% for CA19-9 and the negative predictive value was 52% for CEA and 57% for CA19-9.
In addition, the cut-off values of CEA and CA19-9 used primarily in colorectal disease and pancreatic disease have been applied to gastric cancer.17,18,19,20 Therefore, these current cut-off values have not been properly validated for use in the early detection of gastric cancer recurrence. In this study, we established new cut-off values to improve the low sensitivity of these tumor markers for gastric cancer and to evaluate whether currently used cut-off values of CEA and CA19-9 are appropriate for detection of recurrence.
Consequently, in the case of CEA, there was only a slight change in sensitivity after readjusting the cut-off value. Conversely, in the case of CA19-9, there was an increase in sensitivity of approximately 6% after readjustment of the cut-off value. Even though there were some improvements in sensitivity, further tests are necessary to confirm these results.
Because of the aforementioned limitations, it is important to identify more sensitive and specific tumor markers to detect disease recurrence. However, based on the results of this study, we believe that using the readjusted cut-off value presented here, CA19-9 levels may be useful to monitor and screen for recurrence after radical gastric cancer surgery. Nevertheless, this study was performed retrospectively, so additional prospective studies are needed to confirm these results.
In addition, among patients with elevated preoperative levels of CEA or CA19-9, elevated postoperative CEA and CA19-9 levels had a higher sensitivity for the early detection of recurrence. Expression of CEA and CA 19-9 in gastric cancer, as determined by immunohistochemistry (IHC), was present in 50% to 70% and approximately 30% of cases, respectively.21,22,23 Thus, postoperative IHC staining for CEA and CA19-9 may be an effective and valuable screening tool to detect gastric cancer recurrence. However, IHC staining in all cases of gastric cancer is both ineffective and wasteful. Performing IHC only in cases with normal preoperative and elevated postoperative tumor marker levels would allow for more efficient confirmation of disease recurrence.
On the other hand, we followed patients for 2 years after radical gastric cancer surgery in this study. During that period, 201 (13.3%) patients were lost to follow-up. According to long-term follow up studies, 79% of recurrences after radical gastric cancer surgery occur within 2 years.15,16 In addition, in our hospital in Seoul, South Korea, early gastric cancer patients are followed up every 6 months for 2 years. Moreover, many cases transferred to nearby hospitals after 2 years of follow-up. Therefore, in this study, a follow-up period of 2 years was considered a reasonable amount of time to evaluate the relationship between serum tumor marker levels and recurrence after radical gastric cancer surgery.
In conclusion, measurement of postoperative CEA and/or CA19-9 levels with readjusted cut-off values allows for more effective detection of gastric cancer recurrence. However, our research has limitations: we did not perform a validation of our findings and our data include a low positive predictive value for the new CA19-9 range.
Acknowledgments
This study was supported by research grant from Cancer Research Institute, Seoul National University (2012) and by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1320270).
This study was orally presented at the 85th Annual Meeting of Japanese Gastric Cancer Association and the results were displayed as a poster at the Korea International Gastric Cancer Week 2014, 65th Annual Congress of the Korean Surgical Society.
References
1. Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002; 22:2311–2316. PMID: 12174919.
2. Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, et al. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997; 20:348–353. PMID: 9256887.

3. Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001; 32:41–44. PMID: 11154168.

4. Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000; 16:105–110. PMID: 11381189.

5. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014; 17:26–33. PMID: 23572188.

6. Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001; 181:16–19. PMID: 11248169.

7. Marrelli D, Pinto E, De Stefano A, de Manzoni G, Farnetani M, Garosi L, et al. Preoperative positivity of serum tumor markers is a strong predictor of hematogenous recurrence of gastric cancer. J Surg Oncol. 2001; 78:253–258. PMID: 11745820.

8. Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, et al. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999; 57:55–62. PMID: 10394126.

9. Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001; 22:318–322. PMID: 11553862.

10. Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Cianetti A, et al. The role of serum and gastric juice levels of carcinoembryonic antigen, CA199 and CA724 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998; 124:450–455. PMID: 9750022.

11. Choi SR, Jang JS, Lee JH, Roh MH, Kim MC, Lee WS, et al. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006; 51:2081–2086. PMID: 17009116.

12. Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003; 6:142–145. PMID: 14520526.

13. Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer. 2006; 9:74–81. PMID: 16767361.

14. Qiu MZ, Lin JZ, Wang ZQ, Wang FH, Pan ZZ, Luo HY, et al. Cutoff value of carcinoembryonic antigen and carbohydrate antigen 19-9 elevation levels for monitoring recurrence in patients with resectable gastric adenocarcinoma. Int J Biol Markers. 2009; 24:258–264. PMID: 20082274.

15. Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ, Kim JP, et al. Clinicopathological analysis for recurrence of early gastric cancer. Jpn J Clin Oncol. 2003; 33:209–214. PMID: 12865463.

16. D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004; 240:808–816. PMID: 15492562.
17. Steinberg WM, Gelfand R, Anderson KK, Glenn J, Kurtzman SH, Sindelar WF, et al. Comparison of the sensitivity and specificity of the CA19-9 and carcinoembryonic antigen assays in detecting cancer of the pancreas. Gastroenterology. 1986; 90:343–349. PMID: 2416628.

18. Takasaki H, Uchida E, Tempero MA, Burnett DA, Metzgar RS, Pour PM. Correlative study on expression of CA 19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer patients. Cancer Res. 1988; 48:1435–1438. PMID: 3162196.
19. Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978; 299:448–451. PMID: 683276.

20. Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010; 17:2321–2329. PMID: 20336387.

21. Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002; 15:632–640. PMID: 12065777.

22. Imada T, Rino Y, Takahashi M, Hatori S, Shiozawa M, Tanaka J, et al. Expression of CA19-9, SLX, STN and CEA in relatively early gastric carcinoma. Oncol Rep. 1997; 4:899–904. PMID: 21590162.

23. Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003; 200:39–46. PMID: 12692839.

Fig. 1
Readjusted cut-off values for carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). The curves depict the readjusted cut-off values for CEA (A) and CA19-9 (B) for early detection of recurrence, following radical gastrectomy. AUC = area under the curve; CI = confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download