Abstract
We report our experience of a concurrent robot assisted distal gastrectomy and partial nephrectomy for synchronous early gastric cancer and renal cell carcinoma. A 55-year-old female patient was diagnosed with early gastric cancer on screening endoscopy. Abdominal computed tomography showed an incidental right renal cell carcinoma. Robot assisted distal gastrectomy was performed, followed by partial nephrectomy. The final pathological examination showed signet ring cell carcinoma within the lamina propria and renal cell carcinoma with negative resection margins. The patient showed no evidence of recurrence at 6-months. A robot-assisted combined operation could be a treatment option for early stages of synchronous malignancies.
The use of minimally invasive surgery has become wide spread in many surgical fields. Many studies have demonstrated the feasibility and safety of robot-assisted surgery. Robot-assisted surgery is performed in many hospitals, especially for early stages of cancers.1,2,3 However, reports on robot assisted combined operations are very limited. We report the first successful concurrent robot-assisted distal gastrectomy and partial nephrectomy in a patient with synchronous early gastric cancer and renal cell carcinoma.
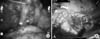
A 55-year-old female patient was diagnosed with early gastric cancer during her annual health screening esophagogastroduodenoscopy. A gastric lesion was identified on the lesser curvature side of the lower body, and endoscopic biopsy confirmed signet ring cell carcinoma. She was previously healthy and, had no medical history of any illness. She was referred to the department of general surgery for further treatment. The patient underwent an abdominal computed tomography scan for further evaluation, and a 3.6-cm sized well-enhancing mass lesion was incidentally found on the lower pole of the right kidney, which was, suggestive of renal cell carcinoma (Fig. 1). The department of urology was consulted. We planned to perform concurrent distal gastrectomy and partial nephrectomy using the da Vinci robot system (Intuitive Surgical, Sunnyvale, CA, USA).
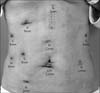
The patient was placed in the supine position under general anesthesia. The general surgery team performed a standard robot-assisted distal gastrectomy. She received preoperative endoscopic clipping to identify the lesion during surgery. A 12-mm camera port was inserted just below the umbilicus via direct vision. After CO2 gas insufflation, three additional 8-mm ports for robotic arms were placed under camera visualization. The ports for arm numbers 1 and 3 were placed in symmetrical fashion, 1 cm below the subcostal margin along both midclavicular lines. The port for arm number 2 was placed 2 cm superior to the umbilicus, between the port for arm number 1 and the camera port. A 12-mm assistant port was inserted 1 cm superior to the umbilicus, between the port for arm number 3 and the camera port (Fig. 2). For liver retraction, a puncture method with a 2-0 Prolene® (Ethicon, Norderstedt, Germany) straight needle was used. After docking the robot, distal gastrectomy was performed in the usual fashion. The operative procedure for distal gastrectomy has been previously described (Fig. 3).2,4,5,6 Complete D2 lymph node dissection around the stomach was performed.
After undocking the robot, a 5-cm vertical midline incision was made, the stomach was removed, and the clips were identified by palpation. After gastric resection, the proximal and distal margins were sent for frozen section examination to confirm negative resection margins. Extracorporeal antecolic gastrojejunostomy was performed using a circular stapler. The incision for mini laparotomy and the port sites in the left abdomen were closed. Other port sites and the abdomen were covered with sterile adhesive drape in case the port sites were reused during partial nephrectomy.
The patient was repositioned to the left lateral decubitus position. The 12-mm umbilical incision was re-used for the assistant port. Then, the 12-mm camera port was inserted 5 cm above the umbilicus to the midline, at the level of the renal hilum. The port for robotic arm number 1 was placed in the 3 finger medial point of the anterior superior iliac spine. The port for arm number 2 was placed in the anterior axillary line at the level of the umbilicus. The port for arm number 3 was placed at the junction of the costal margin and the lateral edge of the rectus muscle. A 5-mm trocar was inserted just below the xiphoid process for liver retraction (Fig. 2). Intraoperative ultrasonography was used to identify the tumor extent. The operative procedure for a partial nephrectomy was performed as previously described (Fig. 3).3,7 The specimen was retrieved using a laparoscopic retrieval bag through the assistant port. Warm ischemia time was 18 minutes. Intraoperative frozen biopsy showed negative resection margins.
The total operation time was 670 minutes (distal gastrectomy, 400 minutes; partial nephrectomy, 270 minutes). The total console time was 370 minutes (distal gastrectomy, 260 minutes; partial nephrectomy, 110 minutes). Estimated blood loss was 300 ml (distal gastrectomy, 100 ml; partial nephrectomy, 200 ml). On postoperative day 5, the patient had a high spiking fever of 39.5℃. A gastrografin upper gastrointestinal series showed no evidence of anastomosis leakage. Abdominal computed tomography showed a complicated fluid collection around the partial nephrectomy site. Intravenous antibiotics were administered and follow-up abdominal computed tomography was performed on postoperative day 13. Computed tomography showed decreased fluid collection around the partial nephrectomy site. The patient was discharged on postoperative day 17. Final pathological examination from the distal gastrectomy showed a 1.6×0.7 cm sized signet ring cell carcinoma, localized within the lamina propria. No metastasis was observed in any of 44 retrieved lymph nodes. The final stage was pT1aN0, stage 1A according to the American Joint Committee on Cancer 7th Edition. Final pathological examination of the partial nephrectomy showed a 3×3 cm sized renal cell carcinoma clear cell type, Fuhrman's nuclear grade II/IV with negative resection margins (Fig. 4). The pathologic stage was T1a. The patient had no evidence of late complications or recurrence at 6 month.
The incidence of synchronous cancer in gastric patients has been reported to be approximately 3%.8,9 Renal cell carcinoma was reported to be the second to fifth most common synchronous malignancy in gastric cancer patients.9,10 Simultaneous surgical resection is the optimal treatment for synchronous malignancies, if possible. For early cancers, using a less invasive surgical technique would benefit the quality of life of these patients.
Robot-assisted surgery was first introduced in 1998 by Himpens et al.,11 who performed a cholecystectomy. Since then, robot assisted surgery has been used in various surgical fields. Robot-assisted distal gastrectomy has been proven to be effective in previous studies.2,4,6 Robotic EndoWrist® (Intuitive Surgical) enables meticulous lymph node dissection, which allows complete D2 lymphadenectomy.2,4,5,6 Partial nephrectomy has been performed to treat incidental small renal masses. Many studies reported the advantages of robot-assisted partial nephrectomy using EndoWrist® in intracorporeal suturing.3,7
Few studies have investigated concurrent robot-assisted resection in urologic malignancies.12,13 To our knowledge, this is the first report describing concurrent robot-assisted resection of gastrointestinal and urologic malignancies. This concurrent robot-assisted operation has the advantage of treating both malignancies in a single operation, thus reducing the morbidity associated with two separate surgeries and hospital admissions. The time and cost of a second operation can be saved for the patient. However this concurrent operation has some drawbacks, particularly the long operation time. As the duration of general anesthesia is longer time than that for each operation alone, special attention is necessary for the patient's positioning to avoid neuromuscular and skin injury. Pneumatic compression devices should be intermittently applied to the patient to prevent deep vein thrombosis during the operation. Patients with underlying comorbidities may not be candidates for combined resection, because of the potential risk of complications with prolonged anesthesia and pneumoperitoneum.
The operation time for distal gastrectomy in this patient was 400 minutes, which is longer than that in previous reports.5,6 The operation time includes initial preparation and docking time for the robot. The patient had a small abdominal cavity, and the distance from the inferior rib margin to the umbilicus was short; thus, docking and adjusting the robotic arm took longer than usual. During left side dissections, the robotic arm number 1 had to be changed to the assistant port entry because of the narrow working space for the robotic arm. Frequent robotic arm adjustments disrupted to the operation flow and caused overall delay.
Recent studies recommend limited lymphadenectomy for early gastric cancer during minimally invasive surgery.5,6 For robotic distal gastrectomy, we perform at least a D1+β dissection. For this case, the preoperative TNM stage was T1aN0M0; however there was a grossly enlarged lymph node along the splenic artery, so we performed a D2 lymph node dissection, that turned out to be negative in the final pathology examination. Meticulous lymph node dissection is a time-consuming procedure, which could have affected the total operation time.
We planned to reuse the ports for partial nephrectomy after distal gastrectomy, leaving the right-sided ports entries open; however, the patient was only 152 cm in height and the renal mass was located on the lower pole, so the distance between the gastrectomy ports was too close. An additional four ports were inserted for partial nephrectomy; only the trocar under the umbilicus was reused. Depending on the tumor location in the kidney, reusing the port could be considered in other cases.
We have demonstrated that combined robot-assisted distal gastrectomy and partial nephrectomy is feasible and safe for the treatment of synchronous early gastric cancer and renal cell carcinoma. For selected patients with early synchronous malignancies, a robot-assisted combined operation could be a minimally invasive treatment option.
Figures and Tables
Fig. 1
Computed tomography showing a 3.6 cm sized enhancing mass lesion in the lower pole of the right kidney.

References
1. Yang HK, Suh YS, Lee HJ. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol. 2013; 107:277–281.

2. Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010; 24:610–615.

3. Kaul S, Laungani R, Sarle R, Stricker H, Peabody J, Littleton R, et al. da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol. 2007; 51:186–191. discussion 191-192.

4. Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009; 249:927–932.

5. Marano A, Hyung WJ. Robotic gastrectomy: the current state of the art. J Gastric Cancer. 2012; 12:63–72.

6. Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging role of robot-assisted gastrectomy: analysis of consecutive 200 cases. J Gastric Cancer. 2013; 13:255–262.

7. Choi JD, Park JW, Lee HW, Lee DG, Jeong BC, Jeon SS, et al. A comparison of surgical and functional outcomes of robot-assisted versus pure laparoscopic partial nephrectomy. JSLS. 2013; 17:292–299.

8. Bae JS, Lee JH, Ryu KW, Kim YW, Bae JM. Characteristics of synchronous cancers in gastric cancer patients. Cancer Res Treat. 2006; 38:25–29.

9. Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008; 98:106–110.

10. Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, et al. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006; 12:2588–2592.

11. Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc. 1998; 12:1091.

12. Patel MN, Eun D, Menon M, Rogers CG. Combined robotic-assisted laparoscopic partial nephrectomy and radical prostatectomy. JSLS. 2009; 13:229–232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download