Abstract
Homeostatic imbalance between cell proliferation and death in gastric mucosal epithelia may lead to gastritis and gastric cancer. Despite abundant gastrokine 1 (GKN1) expression in the normal stomach, the loss of GKN1 expression is frequently detected in gastric mucosa infected with Helicobacter pylori, as well as in intestinal metaplasia and gastric cancer tissues, suggesting that GKN1 plays an important role in gastric mucosal defense, and the gene functions as a gastric tumor suppressor. In the stomach, GKN1 is involved in gastric mucosal inflammation by regulating cytokine production, the nuclear factor-κB signaling pathway, and cyclooxygenase-2 expression. GKN1 also inhibits the carcinogenic potential of H. pylori protein CagA by binding to it, and up-regulates antioxidant enzymes. In addition, GKN1 reduces cell viability, proliferation, and colony formation by inhibiting cell cycle progression and epigenetic modification by down-regulating the expression levels of DNMT1 and EZH2, and DNMT1 activity, and inducing apoptosis through the death receptor-dependent pathway. Furthermore, GKN1 also inhibits gastric cancer cell invasion and metastasis via coordinated regulation of epithelial mesenchymal transition-related protein expression, reactive oxygen species production, and PI3K/Akt signaling pathway activation. Although the modes of action of GKN1 have not been clearly described, recent limited evidence suggests that GKN1 acts as a gastric-specific tumor suppressor. This review aims to discuss, comment, and summarize the recent progress in the understanding of the role of GKN1 in gastric cancer development and progression.
Generally, the gastrointestinal epithelium is characterized by a very high cellular turnover rate, which leads to renewal of the epithelium every 3 to 5 days.1 Continuous processes of cell proliferation, differentiation, and self-renewal are counterbalanced by senescence and/or apoptosis in normal gastric mucosa. To maintain homeostasis, a critical balance between cell proliferation and death is required, and complex signaling pathways and transcriptional regulators control it. Otherwise, homeostatic imbalance, caused by several factors, including infection with Helicobacter pylori, ingested noxious agents, and acidic pH, may lead to diseases such as gastritis and gastric cancer. Therefore, protective and reparative mechanisms are essential to rapidly restore gastric mucosal integrity by stimulating migration of epithelial cells over denuded areas (restitution), increasing mucus production, and reestablishing epithelial proliferation and differentiation programs.2 Although endogenous molecules, such as members of the trefoil factor 1 protein family, protect the gastric mucosa from noxious environments,3 many of their underlying molecular mechanisms have not been clearly elucidated.
Gastrokine 1 (GKN1), also called antral mucosal protein (AMP)-18, is a protein synthesized by the cells of the antral gastric mucosa and shows growth factor or 'cytokine-like' activity toward gastric epithelial cells.4 Immunoelectron microscopy indicated that the GKN1 protein is localized within the granules just under the apical plasma membrane, suggesting that it is a secreted rather than an integral membrane protein.4 Early work reported that GKN1 protects the antral gastric mucosa and promotes healing by facilitating restitution and proliferation following injury.4 Interestingly, this protein is abundantly expressed in the gastric mucosa of healthy individuals, but is down-regulated or absent in gastric cancer tissues.4 Because a disruption in gastric homeostasis may result in the transformation of normal epithelial cells into cancer cells and permit cancer cells to proliferate and invade, there is no doubt that inactivation of GKN1 may render the gastric mucosa vulnerable to carcinogens or gastric injury, and eventually trigger genetic alterations in cancer-related genes, including oncogenes and tumor suppressor genes.
Although limited published evidence suggests that GKN1 may play an important role in gastric epithelial homeostasis and gastric carcinogenesis, its definitive biological functions remain unclear. In this review, we discuss the current understanding of the biological roles of the GKN1 tumor suppressor in the development and progression of gastric cancer.
The protein, previously known as AMP-18, CA11, FOVEOLIN, and TFIZ,5,6,7,8 was formally named 'GKN1' by the HUGO Gene Nomenclature Committee (HGNC no. 23217) for its gastric-specific expression and its highly conserved presence in the gastric mucosa of many mammalian species.5,6 Three members of the GKN family, GKN1, GKN2, and GKN3, are located on chromosome 2p13.3 and arranged within a 60-kb genomic interval.9 The structures of the GKN genes exhibit nearly identical genomic architecture, with each containing six exons separated by relatively short introns.9 GKN genes encode small (from 181- to 184-amino acid) proteins; and contain the enigmatic BRICHOS domain and a COOH-terminal segment showing considerable divergence between the GKN paralogs and the hydrophobic NH2-terminal signal peptide, the processing of which is predicted to generate about 160 mature amino acid proteins with a molecular mass of approximately 18 kDa.9,10 Of these, the NH2-terminal hydrophobic region acts as a transmembrane anchor and/or signal peptide.10 Clinically, the proteins in the BRICHOS superfamily are associated with dementia, respiratory distress syndrome, and cancer.10,11 Molecular studies on the BRICHOS domain function have suggested that it has a range of possible roles, including intracellular trafficking, propeptide processing, chaperone function, and secretion.11 However, the biological activities of the BRICHOS domain in GKN1 have not been elucidated. We previously reported that the NH2-terminal hydrophobic region and BRICHOS domain of GKN1, but not the COOH-terminal region, significantly reduce cell viability, proliferation, and colony formation of AGS gastric cancer cells by regulating cell cycle progression, and minimize epigenetic modification by inhibiting the expression of DNMT1 and EZH2, and DNMT1 activity. Therefore, these findings indicate that the NH2-terminal hydrophobic region and BRICHOS domain of GKN1 might be the main functional domain for tumor suppressor activity.12
Two isoforms of the GKN1 protein with different NH2-terminal residues, aspartic acid (D) and asparagine (N), were found in gastric endoscopy biopsy samples, and the isoform containing asparagine was decreased or absent in some H. pylori-positive patients.13 Therefore, these two isoforms might play different roles in H. pylori-infected gastric mucosa. Previously, we reported that an aspartic acid or asparagine variant at codon 13 in the NH2-terminal region, but not alanine variant of the pGKN1D13A mutant, was found to be important in the anti-growth function of GKN1.12 Therefore, it seems that genetic alterations or loss of expression of the NH2-terminal hydrophobic region and BRICHOS domain of GKN1 might lead to the functional inactivation of the GKN1 protein. However, to explain how GKN1 expression is controlled in normal gastric mucosa, further studies are important to delineate the molecular mechanisms regulating transcription and biological roles of GKN1 in gastric mucosal epithelia.
GKN1 is a unique gastric-specific protein, whose expression is confined to the gastric epithelium, except for trace levels in the uterus and placenta.5 GKN1 protein and mRNA are localized in surface mucous cells of the gastric antrum and fundus in humans.6,13,14,15,16 GKN1 is involved in filling the lumen of the surface layer of epithelial cells to maintain mucosal integrity and regulate cell proliferation and differentiation.5,6 In addition, GKN1 is present in the secretion granules of gastric mucosal epithelial cells during immunogold-labeling experiments4 and, therefore, is predicted to function as a secreted protein acting primarily in the extracellular and luminal environment.9 As mentioned earlier, the gastrointestinal epithelium is characterized by a very high cellular turnover rate, which leads to renewal of the epithelium every 3 to 5 days.1 GKN1 also facilitates regeneration of the injured colonic epithelium by accelerating migration of surviving cells at the edges of wounds, and also stimulating cell proliferation to resurface the injured mucosa after cell detachment, apoptosis, and necrosis.4,5 However, subsequent studies have reported that overexpression of GKN1 inhibits cell proliferation and induces Fas-mediated apoptosis and senescence through p16/Rb pathway activation in gastric epithelial cells.16,17,18,19 Although the molecular mechanisms that explain these opposite effects of GKN1 in colon and gastric epithelial cells remain unclear, it is possible that GKN1 might inhibit or stimulate cell growth in a cell type-specific and context-dependent manner. Therefore, more work is needed to better understand the bioactivity of GKN1 in maintaining the homeostasis of normal gastric mucosa.
Generally, gastric mucosal inflammation is believed to be caused by chronic H. pylori infection. A critical balance between pro- and anti-inflammatory signals maintains a healthy gastric homeostasis, which effectively neutralizes harmful environmental insults to prevent excessive tissue damage.20 Therefore, imbalance between pro- and anti-inflammatory cytokines may render the gastric mucosa vulnerable to injury, resulting in gastritis. Among inflammatory mediators, nuclear factor (NF)-κB is a potent transcriptional factor that orchestrates many biological functions essential for inflammatory and immune processes induced by H. pylori, and whose activation stimulates interleukin-1 (IL-1) and tumor necrosis factor-α production.21,22 In addition to the role of cytokine production, aberrant activation of NF-κB leads to cell growth and resistance to apoptosis in gastric cancer cells.23,24 Interestingly, ectopic GKN1 expression was found to suppress the activation and nuclear translocation of NF-κB by inhibiting degradation and phosphorylation of NF-κB specific inhibitor (IκB) and inactivating IKKα/β in AGS gastric cancer cells (Fig. 1).25
Furthermore, GKN1 induces a significant increase in the expression of IL-17, but markedly decreases the expression of IL-6, IL-10, and cyclooxygenase-2 (COX-2), which are correlated with tumor progression and serve as independent predictors of poor survival in gastric cancer (Fig. 1).25,26,27,28 The IL-17 family of cytokines performs numerous immune regulatory functions related to local tissue inflammation by inducing and mediating proinflammatory responses.29 IL-17 acts upon gastric epithelial cells to release IL-8, which recruits neutrophils,30 and is associated with prolonged survival in gastric cancer patients.31 In contrast, IL-10 is an anti-inflammatory cytokine that suppresses the production of pro-inflammatory cytokines through the inhibition of Th1 lymphocytes and stimulation of B and Th2 lymphocytes, in order to down-regulate the inflammatory response.32 In particular, IL-6 is generally considered to be a pro-inflammatory and pleiotropic cytokine involved in tumor initiation, promotion, and progression.33 COX-2 expression in the injured mucosa plays a significant role in the repair processes and defense mechanisms of the stomach.34 COX-2 inhibition has been shown to suppress cell proliferation and induce apoptosis in many gastrointestinal cancer cell lines.35 Therefore, these findings imply that GKN1 may play an important role in gastric mucosal inflammation by mediating cytokine production and suppression of NF-κB and COX-2, thereby contributing to the maintenance of gastric mucosal homeostasis.
It has been reported that H. pylori infection and long-term non-steroidal anti-inflammatory drug administration down-regulate the expression of GKN1 in gastric mucosal epithelial cells.15,36 In accordance with these observations, the GKN1 mRNA transcript was strongly up-regulated in the gastric transcriptome after H. pylori eradication and cessation of gastritis.37 Previously, we also found that the presence of H. pylori CagA was inversely correlated with GKN1 expression in non-neoplastic gastric mucosa.38 Furthermore, the expression of GKN1 was significantly reduced in the cases with H. pylori CagA positivity, mononuclear cell infiltration, atrophy, and intestinal metaplasia. In addition, GKN1 predicted the risk of atrophy and intestinal metaplasia with an area under the receiver operating characteristic curve value of 0.865 and 0.973, respectively.38 Taken together, these findings provide evidence that the expression levels of GKN1 might be a predictive and diagnostic biomarker for evaluating the severity of gastritis and CagA-positive H. pylori infection.
GKN2, also known as TFIZ1 and Blottin, is expressed in surface pit cells in both the corpus and antrum, and are thought to be secreted.8,39,40,41 GKN2 gene expression was shown to be significantly reduced in AGS and KATO-III gastric adenocarcinoma cell lines by the p50 and p65 subunits of NF-κB, and co-transfection with IκB resulted in up-regulation of the GKN2 gene.2 Interestingly, GKN2 alone did not affect cell viability, whereas co-transfection with GKN1 and GKN2 in vitro showed that GKN2 inhibited the effect of GKN1 on cell viability, proliferation, and apoptosis by down-regulating miR-185 expression and inducing epigenetic modification. Furthermore, the expression of GKN2 was regulated in a GKN1-dependent manner by inactivating the NF-κB signaling pathway.42 Therefore, GKN2 might play an important role in gastric mucosal homeostasis by regulating GKN1 activities. These data agree with the previous findings that GKN2 did not induce any significant effects in the cell wounding assay in HGT-101 gastric cancer cells.40 However, GKN2 expression was also reduced in H. pylori-infected gastritis37 and gastric cancers.43,44 Moreover, GKN2 decreased cell growth in SGC-7901 gastric cancer cells in vitro.43 A recent study confirmed that GKN2 inhibits proliferation, migration, and invasion of gastric cancer cells, and arrests the cell cycle at the G1/S transition phase,44 prompting recognition of GKN2 as a putative stomach-specific tumor suppressor gene and implying its potential use in gastric cancer therapy. While the basis of these controversial results is unclear, we speculate that GKN2 might suppress gastric cancer cell growth, but inhibit GKN1-induced cell death to maintain homeostasis in normal gastric mucosa. Further efforts to clarify the molecular mechanisms underlying GKN2 functions will provide more comprehensive information about the use of GKN2 in anticancer or mucosal protective therapeutics.
GKN1 mRNA is one of the most abundant transcripts in the normal gastric mucosa, accounting for approximately 1% of total gastric mRNA.45 Interestingly, it was demonstrated that individuals with a lower expression of GKN1 have an increased risk of developing gastric disease.14 Numerous studies have described frequent reduction or complete loss of GKN1 expression in gastric tumors, including adenomas and adenocarcinomas.6,7,15,16 Loss of GKN1 expression in gastric cancer was first described in differential display analysis.46 In immunohistochemical analysis, loss of GKN1 expression was detected in 36 (90%) and 170 (89.5%) of gastric adenomas and carcinomas, respectively.16 Statistically, the loss of GKN1 expression in gastric cancer showed no association with tumor differentiation, depth of invasion, location, and lymph node metastasis, or clinical stage.7,16 Therefore, GKN1 inactivation may commonly occur as an early event in the development of gastric tumorigenesis. However, Moss et al.14 revealed the loss of GKN1 expression in 78% of diffuse-type and 42% of intestinal-type gastric cancers, and this loss of expression was positively correlated with that of GKN2. Additionally, the authors found that the combined loss of GKN1 and GKN2 expression may correlate with shorter overall survival in the intestinal-type gastric cancer. Thus, further clinical validation studies in larger populations are necessary to validate the applicability of GKN1 and GKN2 as prognostic and/or differentiation markers for gastric cancer patients.
Although it is well known that GKN1 expression is undetected in gastric tumors, the molecular mechanisms underlying GKN1 inactivation remain unclear. Chromosomal alterations, including deletions or loss of heterozygosity at 2p13.3, where GKN genes are located, have not been found in gastric tumors.47,48,49 Previously, we reported that there was no somatic mutation in the coding regions of GKN1 and the hypermethylation of the GKN1 promoter was observed in 2 (8.0%) of 25 gastric cancers. However, significant loss of the GKN1 gene at DNA and mRNA levels was detected in gastric cancer tissues. Thus, we conclude that DNA and mRNA transcript loss of the GKN1 gene, but not genetic and epigenetic alterations, may play a major role in the loss of GKN1 expression in gastric cancer.16
Chronic infection with H. pylori perpetuates chronic gastritis, which progresses to mucosal atrophy, intestinal metaplasia, dysplasia, and carcinoma.50 As described above, GKN1 expression is down-regulated in H. pylori-infected gastric mucosal tissue.13,15,36,37 These observations invoke the hypothesis that H. pylori infection might regulate the expression of GKN1 protein. In recognition of this possibility, we analyzed the effects of H. pylori CagA on GKN1 expression in gastric epithelial cells. Interestingly, H. pylori CagA-induced reactive oxygen species (ROS) reduced the expression of the GKN1 protein by decreasing GKN1 DNA and mRNA copy numbers in gastric cell lines and mucosal tissues of humans and mice.51 As shown in Fig. 1, the ectopic expression of GKN1 inhibited CagA-induced ROS production and GKN1 copy number change via overexpression of antioxidant enzymes, MnSOD, and catalase. In addition, GKN1 prevented the injection of CagA into cells by binding to CagA at the extracellular level,51 suggesting that GKN1 may counteract the CagA-induced genetic alterations. These results were corroborated by those of Moss and colleagues,14 who reported that individuals with a lower expression of GKN1 have an increased risk of developing gastric cancer. Therefore, it is expected that the presence of GKN1 helps to maintain the homeostasis of normal gastric mucosa, even if H. pylori infection exists. In contrast, inactivation of the GKN1 gene may lead to a defect in the gastric mucosal barrier, rendering gastric mucosa subject to exposure to carcinogens, including H. pylori CagA, and eventually evoke subsequent genetic alterations of tumor suppressor genes or oncogenes involved in gastric carcinogenesis.
Epithelial mesenchymal transition (EMT) is a normal physiologic process, during which cells lose epithelial characteristics and gain more motile mesenchymal features allowing relocation of the cells to new sites and participation in tissue and organ formation.52 This phenomenon is observed in response to injury, organ fibrosis, and cancer. In cancers originated from epithelial cells, EMT converts epithelial cells into cells with invasive characteristics that enable them to destroy and move into surrounding normal tissues, including blood and lymphatic vessels, and to spread systemically into other tissues or organs. In addition, circulating tumor cells are thought to undergo EMT, whereby epithelial traits are lost in favor of more mesenchymal traits, thus acquiring invasive capabilities and plasticity.53 Unfortunately, most gastric cancer patients are diagnosed at the advanced stages of the disease, which contribute to the high lethality rate in the world.54 The identification of signals that lead to EMT and cancer cell migration remains a central challenge in cancer research, and a better understanding of the molecular mechanisms of invasion and metastasis of gastric cancer may provide new insight and information to develop novel therapeutic targets for treatment of gastric cancer patients.
As described above, turning epithelial cancer cells into mesenchymal cells was proposed to be the critical process for the acquisition of the invasive and metastatic phenotype of epithelial cancer cells during tumor progression. Previously, it was reported that ROS stimulated EMT,55 and the activation of PI3K/Akt signaling was detected in cells undergoing EMT.56 Our subsequent study found that GKN1 induced the conversion of spindle-shaped cells with abundant cytoplasms to circular-shaped cells, and suppressed cell migration and invasiveness in wound healing, transwell chemotaxis, and invasion assays.57 In addition, GKN1 significantly reduced ROS levels by up-regulating antioxidant enzymes, including MnSOD and catalase, and completely abrogated expression of the PI3K/Akt pathway proteins, concomitant with the re-expression of E-cadherin and decreased expression of fibronectin, vimentin, nuclear β-catenin, slug, snail, and cyclin D1 (Fig. 1).57 This limited evidence indicates that GKN1 might inhibit the invasion and metastasis of gastric cancer cells via coordinate regulation of expression of EMT-related proteins, ROS production, and the activation of the PI3K/Akt signaling pathway. Based on the above data, we conclude that GKN1 may inhibit not only the development, but also the progression of gastric cancer.
Numerous studies have described frequent loss of GKN1 expression in gastric cancer and its anti-proliferative activity in gastric epithelial cells, suggesting that GKN1 may be a gastric specific tumor suppressor. However, the definitive biological functions of GKN1 have not yet been identified. In colonic epithelial cells, GKN1 exerted its protective effects by increasing accumulation of specific tight and adherence junction proteins, and protecting their loss after injury.58 Toback et al.4 also proposed that the GKN1 protein could have some mitogenic impacts on intestinal epithelial cells. In contradiction to its protective and mitogenic activity, Shiozaki et al.7 found that GKN1 was capable of inhibiting the proliferation of MKN28 cells after transfection. Similarly, subsequent studies demonstrated that GKN1 not only inhibited cell proliferation, but also induced apoptosis and senescence.16,17,18,19,59,60 As expected, restoration of GKN1 expression resulted in cell cycle arrest at the G1/S or G2/M phases by down-regulating positive cell cycle regulators, including CDK4, cyclin D1, E2F, cdc25, and cyclin B, and up-regulating negative cell cycle regulators, including p16 and p21 (Fig. 1).17,18,61 Using proteomics analysis, Yan et al.61 identified the pro-oncogenic protein enolase-α (ENO1), which prevented GKN1-dependent growth inhibition and cell cycle arrest and, thus, might be a possible target and antagonist of GKN1 activity in gastric cancer cells. Recently, we examined miRNA-185 to elucidate the underlying molecular mechanisms of GKN1-dependent growth inhibition. We demonstrated that GKN1 suppressed gastric cancer cell growth by inducing endogenous miR-185 that directly targeted epigenetic effectors, such as DNMT1, EZH2, and HDAC (Fig. 1).17 In addition, GKN1 expression was inversely correlated with DNMT1 and EZH2 expression in gastric cancer tissues and various gastric cancer cell lines. Furthermore, RNA interference-mediated knockdown of GKN1 in HFE-145 non-neoplastic gastric epithelial cells, which express GKN1 protein, induced cell growth, while GKN1 was sufficient to induce cell cycle arrest in AGS, MKN1, and MKN28 gastric cancer cells. Moreover, GKN1-induced miR-185 converted hypermethylated CDKN2A and E-cadherin to the unmethylated forms in AGS cells (Fig. 1).17 Therefore, these results suggest that GKN1 may inhibit gastric carcinogenesis through the induction of miR-185, thereby modulating epigenetic alterations in cell cycle regulatory components.
Regarding cell death, ectopic expression of GKN1 in AGS gastric cancer cells induced activation of the apoptosis-related proteins, including cleaved caspase-8, caspase-3, and poly-(ADP-ribose) polymerase, whereas no change in expression of the mitochondrial pathway-related proteins was observed (Fig. 1).16 Thus, we conclude that GKN1 may induce apoptosis via the death receptor-mediated, but not the mitochondria-mediated pathway. Similarly, Rippa et al.19 reported that AGS cells overexpressing GKN1 showed higher expression of Fas receptor and sensitivity to Fas-ligand-induced apoptosis. In addition, Chen et al.62 identified the cholecystokinin-B/gastrin receptor (CCKBR) as a putative receptor for GKN1 protein by affinity purification and mass spectrometry analysis. The authors found that recombinant human AMP-18 (GKN1) bound to the plasma membrane of keratinocytes in normal human oral mucosal tissue, and that CCKBR was co-immunoprecipitated with exogenous AMP-18 in diverse epithelial cells. In gastric epithelial cells, binding of gastrin to CCKBR induced the expression and release of heparin-biding epidermal-like growth factor, which subsequently transactivated the epithermal growth factor receptor and downstream signaling pathways.63 However, no data has been reported concerning the existence of a receptor for secretary GKN1 at the membrane of gastric mucosal epithelial cells, to which GKN1 may directly bind to. Thus, to investigate whether GKN1 directly binds to CCKBR at the membrane of gastric epithelial cells, and if so, how GKN1 regulates the complex of gastrin and CCKBR and maintains the gastric mucosal homeostasis will be of great interest to study further.
Recent evidence has demonstrated that GKN1 may play an important role in the maintenance of gastric mucosal homeostasis and function as a gastric specific tumor suppressor. Although the molecular mechanisms underlying its tumor suppressor activity are largely unknown, it was found that GKN1 is involved in gastric mucosal inflammation by regulating production of inflammatory mediators, including NF-κB, COX-2, and cytokines. In addition, GKN1 inhibits the carcinogenic potentials of H. pylori CagA through the direct binding to CagA at the extracellular level, and increases the expression of antioxidant enzymes at the intracellular level. Furthermore, GKN1 interferes with cell growth and invasion by suppressing cell cycle progression and EMT, and induces apoptosis via the death receptor-dependent pathway, thereby inhibiting not only the development, but also the progression of gastric cancer. Thus, it is plausible that modulating GKN1 activity and stimulating its anti-cancerous effects could significantly influence the development of novel cancer therapeutics, which would ultimately achieve the goal of gastric cancer prevention and remission. Future studies to clarify the biological activity and regulatory mechanisms of GKN1 will be pivotal to develop GKN1 as a potential therapeutic target for gastric cancer patients.
Figures and Tables
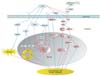 | Fig. 1Role of GKN1 in gastric cancer. GKN1 induces apoptosis through the death receptor dependent pathway and inhibits Helicobacter pylori CagA injection into gastric epithelial cells. GKN1 also reduces reactive oxygen species production by up-regulating the expression of antioxidant enzymes, such as MnSOD and catalase. At the intracellular level of gastric epithelial cells, GKN1 induces miR-185 expression by down-regulating c-myc, which in turn inhibits epigenetic modification of genomic DNA and stimulates the expression of negative cell cycle regulators, including p53, p21, and p16. In addition, GKN1 inhibits epithelial mesenchymal transition by inactivating PI3K/Akt and β-catenin signaling pathway, resulting in the disruption of invasion and metastasis of gastric cancer cells. Blue line: stimulator, Red line: inhibitor. GKN1 = gastrokine 1; ROS = reactive oxygen species; NF-κB = nuclear factor-κB; COX2 = cyclooxygenase-2; CASP3 = caspase 3; CASP8 = caspase 8; CDH1 = cadherin 1; DNMT1 = DNA methyltransferase 1; EZH2 = enhancer of zeste homolog 2; PI3K = phosphatidylinositol 3-kinase; IKKα/β = IκB kinase α/β. |
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A2A01002531).
References
1. Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994; 107:3569–3577.

2. Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine regulation of the trefoil factor family binding protein GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial cells. Cell Physiol Biochem. 2007; 20:193–204.

3. Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996; 110:489–497.

4. Toback FG, Walsh-Reitz MM, Musch MW, Chang EB, Del Valle J, Ren H, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003; 285:G344–G353.
5. Martin TE, Powell CT, Wang Z, Bhattacharyya S, Walsh-Reitz MM, Agarwal K, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003; 285:G332–G343.
6. Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004; 203:789–797.

7. Shiozaki K, Nakamori S, Tsujie M, Okami J, Yamamoto H, Nagano H, et al. Human stomach-specific gene, CA11, is down-regulated in gastric cancer. Int J Oncol. 2001; 19:701–707.

8. Westley BR, Griffin SM, May FE. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry. 2005; 44:7967–7975.

9. Menheniott TR, Kurklu B, Giraud AS. Gastrokines: stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am J Physiol Gastrointest Liver Physiol. 2013; 304:G109–G121.

10. Hedlund J, Johansson J, Persson B. BRICHOS - a superfamily of multidomain proteins with diverse functions. BMC Res Notes. 2009; 2:180.

11. Sánchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci. 2002; 27:329–332.

12. Yoon JH, Choi YJ, Choi WS, Nam SW, Lee JY, Park WS. Functional analysis of the NH2-terminal hydrophobic region and BRICHOS domain of GKN1. Biochem Biophys Res Commun. 2013; 440:689–695.

13. Nardone G, Rippa E, Martin G, Rocco A, Siciliano RA, Fiengo A, et al. Gastrokine 1 expression in patients with and without Helicobacter pylori infection. Dig Liver Dis. 2007; 39:122–129.

14. Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, et al. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008; 14:4161–4167.

15. Nardone G, Martin G, Rocco A, Rippa E, La Monica G, Caruso F, et al. Molecular expression of Gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic gastric lesions. Cancer Biol Ther. 2008; 7:1890–1895.

16. Yoon JH, Song JH, Zhang C, Jin M, Kang YH, Nam SW, et al. Inactivation of the Gastrokine 1 gene in gastric adenomas and carcinomas. J Pathol. 2011; 223:618–625.

17. Yoon JH, Choi YJ, Choi WS, Ashktorab H, Smoot DT, Nam SW, et al. GKN1-miR-185-DNMT1 axis suppresses gastric carcinogenesis through regulation of epigenetic alteration and cell cycle. Clin Cancer Res. 2013; 19:4599–4610.

18. Xing R, Li W, Cui J, Zhang J, Kang B, Wang Y, et al. Gastrokine 1 induces senescence through p16/Rb pathway activation in gastric cancer cells. Gut. 2012; 61:43–52.

19. Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011; 226:2571–2578.

20. Guang W, Ding H, Czinn SJ, Kim KC, Blanchard TG, Lillehoj EP. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J Biol Chem. 2010; 285:20547–20557.

21. Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, et al. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000; 95:2768–2776.
22. Sharma SA, Tummuru MK, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998; 160:2401–2407.
23. Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008; 135:2030–2042.

24. Liu CA, Wang MJ, Chi CW, Wu CW, Chen JY. Rho/Rhotekin-mediated NF-kappaB activation confers resistance to apoptosis. Oncogene. 2004; 23:8731–8742.

25. Yoon JH, Cho ML, Choi YJ, Back JY, Park MK, Lee SW, et al. Gastrokine 1 regulates NF-κB signaling pathway and cytokine expression in gastric cancers. J Cell Biochem. 2013; 114:1800–1809.

27. Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu AS, et al. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008; 14:428–434.

28. Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009; 12:95–100.

29. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008; 226:57–79.

30. Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter. 2011; 16:1–8.

31. Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, et al. Prognostic significance of IL-17 mRNA expression in peritoneal lavage in gastric cancer patients who underwent curative resection. Oncol Rep. 2014; 31:605–612.

32. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991; 174:1209–1220.

33. Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007; 110:1911–1928.
34. Gudis K, Sakamoto C. The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci. 2005; 50:Suppl 1. S16–S23.

35. Grösch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006; 98:736–747.

36. Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Helicobacter pylori infection and administration of non-steroidal anti-inflammatory drugs down-regulate the expression of gastrokine-1 in gastric mucosa. Turk J Gastroenterol. 2012; 23:212–219.

37. Resnick MB, Sabo E, Meitner PA, Kim SS, Cho Y, Kim HK, et al. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut. 2006; 55:1717–1724.

38. Choi WS, Seo HS, Song KY, Yoon JH, Kim O, Nam SW, et al. Gastrokine 1 expression in the human gastric mucosa is closely associated with the degree of gastritis and DNA methylation. J Gastric Cancer. 2013; 13:232–241.

39. Kouznetsova I, Laubinger W, Kalbacher H, Kalinski T, Meyer F, Roessner A, et al. Biosynthesis of gastrokine-2 in the human gastric mucosa: restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell Physiol Biochem. 2007; 20:899–908.

40. Otto WR, Patel K, McKinnell I, Evans MD, Lee CY, Frith D, et al. Identification of blottin: a novel gastric trefoil factor family-2 binding protein. Proteomics. 2006; 6:4235–4245.

41. Otto WR, Thim L. Trefoil factor family-interacting proteins. Cell Mol Life Sci. 2005; 62:2939–2946.
42. Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, et al. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014; 229:762–771.

43. Du JJ, Dou KF, Peng SY, Wang WZ, Wang ZH, Xiao HS, et al. Down-regulated full-length novel gene GDDR and its effect on gastric cancer. Zhonghua Yi Xue Za Zhi. 2003; 83:1166–1168.
44. Dai J, Zhang N, Wang J, Chen M, Chen J. Gastrokine-2 is downregulated in gastric cancer and its restoration suppresses gastric tumorigenesis and cancer metastasis. Tumour Biol. 2014; 35:4199–4207.

45. Oien KA, Vass JK, Downie I, Fullarton G, Keith WN. Profiling, comparison and validation of gene expression in gastric carcinoma and normal stomach. Oncogene. 2003; 22:4287–4300.

46. Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000; 91:459–463.

47. Chetty R, Naidoo R, Tarin M, Sitti C. Chromosome 2p, 3p, 5q and 18q status in sporadic gastric cancer. Pathology. 2002; 34:275–281.

48. Panani AD. Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett. 2008; 266:99–115.

49. Noguchi T, Wirtz HC, Michaelis S, Gabbert HE, Mueller W. Chromosomal imbalances in gastric cancer. Correlation with histologic subtypes and tumor progression. Am J Clin Pathol. 2001; 115:828–834.
50. Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003; 3:592–600.

51. Yoon JH, Seo HS, Choi SS, Chae HS, Choi WS, Kim O, et al. Gastrokine 1 inhibits the carcinogenic potentials of Helicobacter pylori CagA. Carcinogenesis. 2014; in press.

52. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–890.

53. Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012; 22:361–368.

54. Durães C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014; 464:367–378.

55. Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005; 436:123–127.

56. Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005; 24:7443–7454.

57. Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011; 137:1697–1704.

58. Walsh-Reitz MM, Huang EF, Musch MW, Chang EB, Martin TE, Kartha S, et al. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005; 289:G163–G171.

59. Pavone LM, Del Vecchio P, Mallardo P, Altieri F, De Pasquale V, Rea S, et al. Structural characterization and biological properties of human gastrokine 1. Mol Biosyst. 2013; 9:412–421.

60. Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Down-regulation of gastrokine-1 in gastric cancer tissues and restoration of its expression induced gastric cancer cells to apoptosis. J Exp Clin Cancer Res. 2012; 31:49.

61. Yan GR, Xu SH, Tan ZL, Yin XF, He QY. Proteomics characterization of gastrokine 1-induced growth inhibition of gastric cancer cells. Proteomics. 2011; 11:3657–3664.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download