Case Report
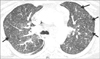
A 41-year-old previously healthy woman presented to the hospital with dyspnea on exertion and dry cough for 1 week. A computed tomography (CT) scan of the chest revealed small mediastinal lymph nodes. The patient was discharged on a tapering dose of oral steroids and albuterol inhalation with a follow-up outpatient appointment with a pulmonologist. An endobronchial ultrasound-guided biopsy of the mediastinal lymph nodes was performed 1 week later. The patient had worsening dyspnea and was again admitted to the hospital. She denied any orthopnea or paroxysmal nocturnal dyspnea. On admission, the physical examination, including chest auscultation, was unremarkable. Initial basic blood work, including complete cell count and metabolic profile, revealed no abnormalities. Her serum cardiac brain natriuretic peptide level was elevated at 1,500 U/L, along with mild elevation of cardiac troponin. The chest radiograph was unremarkable. A CT scan of the chest revealed new small nodular opacities with a tree in bud appearance in the peripheral centrilobular area (
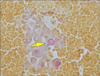
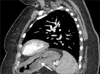
Fig. 1). Subsequent transthoracic echocardiogram revealed extremely elevated right ventricular systolic pressures with flattening of the interventricular septum and severely high pulmonary artery pressures, suggestive of acute cor pulmonale from severe pulmonary hypertension. Within a day of admission, the patient's dyspnea worsened and she required high-flow oxygen. Meanwhile, mediastinal lymph node biopsy revealed signet ring cell poorly differentiated adenocarcinoma (
Fig. 2), and mucicarmine staining confirmed mucin secreting signet ring cells (
Fig. 3). A CT scan of the abdomen was performed to look for the primary tumor, and revealed a diffusely thickened stomach wall with enhancement consistent with diffuse gastric cancer (
Fig. 4), in accordance with the signet ring adenocarcinomatous cells observed in the lymph node biopsy. At that time, the patient's plasma prothrombin time was elevated at 80 seconds, and further testing showed a plasma D-dimer level of more than 5,000 µg/L with a low fibrinogen level of 100 mg/dl, suggestive of disseminated intravascular coagulation (DIC). The CT scan appearance of the chest, acute severe right-sided heart failure from severe pulmonary hypertension, and DIC were characteristic of PTTM. Accordingly, the patient was started on empiric anticoagulation with heparin and intravenous corticosteroids for PTTM based on anecdotal case reports. The patient was scheduled to receive chemotherapy for the gastric adenocarcinoma; however, the hypoxia worsened and the patient required ventilator support. Unfortunately, she rapidly progressed to death within an hour due to extreme hypoxia and cardiac arrest. The patient's family declined autopsy.
Since diffuse gastric cancer is associated with hereditary diffuse gastric cancer (HDGC), it was important to know if the patient's three daughters were at risk. The updated guidelines recommend testing for cadherin 1 (CDH1) gene mutation in a patient's blood when the diagnosis of diffuse gastric cancer occurs at the age of 40 years or less. Thus, we decided to test for the CDH1 genetic mutation in the patient's stored blood. Fortunately, the test came back negative, ruling out HDGC in principle.
Discussion
Gastric cancer remains the second most common cause of cancer-related deaths worldwide.
3,
4,
5 Gastric carcinoma cells have a high tendency to metastasize to the lymph nodes and distant organs,
6 which is likely strongly associated with its poor prognosis.
7 In particular, carcinoma cells in the pulmonary artery significantly influence the risks of tumor recurrence and death after resection.
8 Moreover, these cells may also cause remodeling, especially asymmetric thickening of the intima of the pulmonary artery, which in turn may induce an increase in the pressure of the right ventricle.
One condition of remodeling is PTTM. PTTM, first described by von Herbay et al.
1 in 1990, is a rare but fatal pulmonary complication in patients with cancer, and is associated with a high risk of severe pulmonary hypertension. It has been found to be more frequently associated with gastric cancer compared to other types of cancers. Histologically, PTTM is characterized by fibrocellular intimal proliferation of small pulmonary arteries and arterioles in patients with metastatic carcinoma.
1 In a study by von Herbay et al.,
1 in which the authors examined 630 consecutive autopsy cases of patients with cancer, PTTM was observed in 21 cases. Interestingly more than half (n=11) of these cases were gastric carcinoma.
To date, fewer than 80 cases of PTTM have been reported in the literature in the MEDLINE database. In a recent study by Fujishiro et al.,
9 103 cases were identified in MEDLINE and the Japan Medical Abstracts Society up until March 2013, and, in accordance with previous studies, the authors found that the most frequent primary cancer complicated by PTTM was gastric cancer (58 cases).
The details of the pathophysiology of this condition are sparse, but a proposed mechanism of PTTM is that tumor cells might not only occlude the small arteries and arterioles, but also activate the coagulation system and release inflammatory mediators and growth factors, including VEGF, thereby resulting in thrombosis, fibrocellular intimal proliferation, and luminal stenosis.
10,
11 The reason for the higher incidence of PTTM in gastric cancer is unknown, and previously published reports have not been focused on explaining this high association. However, we hypothesize that it may be related to the high VEGF expression in gastric cancer
12 and its direct influence on hematogenous metastases, which in turn eventually activates a coagulation cascade in the pulmonary arteries. High VEGF expression is characteristic of gastric cancer.
12 Expression of VEGF, serum VEGF, and other proangiogenic factors were studied by Oh et al.,
13 who examined 114 specimens of gastric cancer obtained by curative gastrectomies. As determined by immunohistochemical analysis, high VEGF expression was seen in 57.9% of the specimens. VEGF is one of the growth factors released by gastric cancer cells, and forms a feedback loop to the cancer cells, in addition to inducing angiogenesis in the endothelial cells. In a study by Fondevila et al.,
14 VEGF expression was found to be associated with a shorter survival time and high microvessel density in gastric cancer, and Tanigawa et al.
15 accordingly found that a high level of microvessel density was associated with an increased risk of hematogenous metastases.
In a recent study of autopsy findings in gastric cancer by Ishiwatari et al.,
16 cancer cells were present in the pulmonary arteries in 30.4% (51/168) of the cases, and this is known to increase the risk of cancer cell implantation in vessels and thereby the risk of developing of PTTM. In addition, diffuse-type gastric carcinoma may be apt to cause remodeling of the pulmonary artery.
It is extremely difficult to diagnose PTTM, and most of the patients rapidly succumb to death due to severe respiratory failure. In a recent case series by Fujishiro et al.,
9 only 7 out of 103 reported cases of PTTM (reported between 1990 and March 2013) were pathologically diagnosed with PTTM while alive. At the same time, it is expected that making a pathological diagnosis by either pulmonary artery catheterization or transbronchial lung biopsy in the setting of extreme hypoxia is difficult. Accordingly, in their study, the mean duration of the period between onset of symptoms to admission was only approximately 1 month, and the median survival of the patients was only 5 days from admission.
9
Patients typically present with dyspnea and physical findings consistent with pulmonary hypertension and right heart failure. Typically, there is evidence of metastatic disease at the time of presentation, but cases of occult cancer manifested as pulmonary thrombotic microangiopathy have been reported. Moreover, more than half of the cases (55.6%) are eventually complicated with DIC.
17
We here describe the radiological features of PTTM, which may aid in the early diagnosis of this condition. Most of the cases reported in the literature were diagnosed post mortem, and these previous studies did not report on the radiological features of this condition. We believe that considering these features may help in the early diagnosis and timely aggressive therapeutic intervention of PTTM. A tree in bud pattern on thin-section CT, which is characterized by small centrilobular nodules and branching linear opacities, is most often seen in patients with infectious bronchiolitis;
18 however, Franquet et al.
19 for the first time reported a tree in bud appearance in a patient with PTTM upon CT. In PTTM, the tree-in-bud pattern largely results from fibrocellular intimal hyperplasia in the small arteries and arterioles. Thus, the cause of the tree-in-bud pattern upon CT is vascular. One possible way to differentiate between an infectious cause and a vascular cause is that the branching linear opacities are in continuation with the bronchioles when multiple images are viewed in the case of infectious bronchiolitis. However, in the case of PTTM, the tree-in-bud pattern is not a continuation of the terminal bronchioles and rather stands out separate.
The above-mentioned clinical and radiological features, as well as laboratory findings showing prolonged prothrombin and activated partial thromboplastin times, low fibrinogen levels, and markedly elevated D-dimer, consistent with DIC, are helpful in the clinical diagnosis of PTTM, since it may not always be possible to pursue a lung biopsy in every patient depending on the situation.
A definitive treatment for PTTM has not been established since most of the cases are diagnosed on autopsy. Based on the few anecdotal case reports, chemotherapy, corticosteroids, and anticoagulation therapy may be beneficial if administered at an early stage. Type 2A serotonin receptor antagonists may be useful by their action of suppressing intimal proliferation; further, drugs for the treatment of primary pulmonary arterial hypertension, such as endothelin antagonists, prostacyclin analogues, and phosphodiesterase type 5 inhibitors may be beneficial, but have not yet been studied. Moreover, one interesting focus will be the use of VEGF-inhibitors, since VEGF is a major mediator of the coagulation cascade and intimal proliferation in PTTM.
All clinicians should be aware that PTTM can cause rapidly worsening respiratory conditions in patients with cancer, especially gastric cancer. To our knowledge, only two previous cases have shown successful outcomes in patients with gastric cancer, owing to early recognition and early administration of chemotherapy, corticosteroids, anticoagulation, antiplatelet agents, and certain anti-cancer agents such as fluoropyrimidines.
20,
21 High clinical suspicion of this condition is necessary, and may improve the clinical outcomes.
Diffuse gastric cancer, also known as signet ring carcinoma, is a poorly differentiated adenocarcinoma that infiltrates into the stomach wall, causing thickening of the wall (
linitis plastica) without forming a distinct mass. HDGC is a subgroup of diffuse gastric cancer.
22 The average age of onset of HDGC is 38 years, and the CDH1 gene mutation is the only genetic mutation described so far that has been associated with the occurrence of HDGC (25%~50% of cases).
23 The recent consensus guidelines in 2010 have broadened the criteria of testing for the CDH1 gene mutation as follows:
23
Histological confirmation of diffuse gastric cancer is only required for one family member
Inclusion of individuals with diffuse gastric cancer before the age of 40 years without a family history
Inclusion of individuals and families with diagnoses of both diffuse gastric cancer (including one before the age of 50 years) and lobular breast cancer
If positive, their offspring should subsequently be tested for the mutation as it has a high penetrance rate of 70% to 80%. In mutation-positive individuals, prophylactic total gastrectomy at a center of excellence should be strongly considered, as a systematic histological study of prophylactic gastrectomies almost universally showed pre-invasive lesions, including in situ signet ring carcinoma.
23 Our patient almost met the second criteria and since she had three children, we pursued genetic testing of the patient's serum. Fortunately, the test was negative. Thus, it is important to consider the possibility of HDGC in similar patients.
In conclusion, PTTM is a lethal condition that tends to be frequently associated with gastric cancer especially poorly differentiated diffuse gastric cancer. It is important to increase the awareness of this condition and have high a clinical suspicion of this entity in appropriate setting as early diagnosis may improve the mortality from this fatal condition.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download