Abstract
Gastric metastasis from ovarian cancer is rarely reported worldwide. In Korea, only 2 such cases have been reported. Here we report a case of a 58-year-old woman with metastatic gastric cancer from an ovarian adenocarcinoma. Endoscopic examination showed that the cancer presented as a submucosal tumor without ulceration. A subsequent gastrectomy confirmed the diagnosis of metastatic ovarian serous adenocarcinoma.
Although ovarian cancer usually spreads along the peritoneum and throughout the pelvic and abdominal cavities, gastrointestinal involvement is uncommon.1 Metastatic gastric cancer from ovarian cancer is extremely rare.2 In Korea, only 2 instances of gastric metastasis from ovarian adenocarcinoma have been described.2,3 Here we report an additional case that presented as a submucosal tumor without ulceration during an endoscopic ultrasound (EUS) and endoscopic examination. The diagnosis of metastatic ovarian cancer was confirmed by post-gastrectomy histopathological examination.
In January 2014, a 58-year-old woman was admitted to our department with a gastric submucosal tumor that was recently discovered during a routine checkup and gastroduodenoscopy. She did not complain of any symptoms. Her medical history showed that she had undergone a total thyroidectomy due to thyroid cancer in 2009, as well as wide excision and radiation treatment for ductal carcinoma in situ of the breast in 2013.
More relevant to the present case, the patient was diagnosed with ovarian serous adenocarcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage IIIB) in 2011. She underwent a total hysterectomy with salpingo-oophorectomy and pelvic and paraaortic lymph node dissection with total omentectomy, followed by 6 cycles of adjuvant chemotherapy with carboplatin and paclitaxel. In 2012, the patient was re-examined and found to have newly developed seeding metastases in the perihepatic space, omentum, perirectal area, diaphragm, and external iliac chain. Her CA-125 level had increased to 51.06 U/ml. Following a debulking operation, she received 9 additional cycles of adjuvant chemotherapy with the previously described regimen. Subsequently, the CA-125 levels were tested every 3 months. After the additional chemotherapy, it had decreased to the normal range and a radiologic examination revealed tumor regression.
The gastric submucosal tumor found in January 2014 was a 2 cm low-echogenic lesion located on the greater curvature of the antrum and showed irregularity in the propria muscle layer (Fig. 1). The pathological diagnosis from the endoscopic biopsy before the operation was chronic active gastritis.
A computed tomography (CT) scan indicated focal thickening and the formation of a mass in the greater curvature of the gastric body, as well as multiple enlarged lymph nodes along the greater curvature of the stomach (Fig. 2).
We considered several possible diagnoses, such as metastatic lesions deriving from the ovary or breast. The patient subsequently underwent curative subtotal gastrectomy with lymphadenectomy. During the operation, multiple enlarged lymph nodes were found to be conglomerated with the stomach, but no other recurrent tumors were found. Frozen section biopsy revealed that the lymph nodes were malignant. Macroscopically, a 3.5×3.0 cm submucosal tumor with ulceration was located in the stomach (Fig. 3). Microscopically, infiltration of metastatic serous adenocarcinoma cells into normal gastric tissues was observed. A total of 28 out of 48 retrieved perigastric lymph nodes were found to be metastatic. A cytologic examination revealed the absence of cancer cells. Immunohistochemical staining revealed that the tumor cells were positive for WT-1, and negative for GCDPF-15 and CD 117 (Fig. 4). The final diagnosis was gastric metastasis from ovarian serous adenocarcinoma. The patient's postoperative course was unremarkable and she was discharged 9 days after the operation. She will undergo another round of chemotherapy with carboplatin plus gemcitabine in consultation with gynecologists.
Metastatic disease involving the stomach is unusual. Most gastric metastases arise from primary breast cancer, followed by melanoma and lung cancer. The incidence of gastric metastasis is 3.6% in patients with breast cancer and 1.3% in patients with lung cancer.1 In Korea, several cases of gastric metastasis from breast cancer, lung cancer, and cholangiocarcinoma, among others, have been reported.4,5,6 The clinical manifestations of metastasis to the stomach vary, but include epigastric pain, melena, anemia from occult gastrointestinal blood loss, nausea, and vomiting. However, our patient did not complain of any symptoms.
Ovarian tumors comprise only 0.013% to 1.6% of all gastric metastatic tumors.7 Reports worldwide of gastric metastases from ovarian cancer are rare. Ovarian carcinomas are more likely to metastasize along the peritoneal surface due to exfoliating cells implanted throughout the peritoneum. In fact, the intraperitoneal route is considered to be the most common route of dissemination.7 However, in the present case, there was no evidence of peritoneal metastasis after preoperative studies, including an abdominal CT scan. We carefully examined the whole abdominal cavity to rule out seeding. The surface of the stomach was normal, except for multiple enlarged perigastric lymph nodes. Ovarian carcinoma can metastasize to other organs, including the stomach, through the blood or lymphatic stream without any evidence of peritoneal metastasis, as in the present case. Although the mechanism of gastric metastasis remains unclear, it can be explained at least in part by the rich blood supply of the stomach.1
A number of instances of metastatic ovarian cancer that presented, similarly to the present case, as single submucosal gastric lesions have been reported. Zhou and Miao1 reviewed all recorded cases of gastric metastasis from ovarian carcinoma and found that among 1,010 patients with malignant tumors 17 had metastases in the stomach, which represents a frequency of 1.7%. Another series of autopsies discovered 92 cases of gastric metastases among a total of 7,165 cases, which is a rate of 1.28%. The prognosis for ovarian carcinoma with gastric metastases remains unknown, although Zhou and Miao1 reported a 1-year survival rate of 83.33% (5 out of 6 cases).
Jung et al.2 reported a case of primary ovarian carcinoma with gastric metastasis and suggested that submucosal tumors of the stomach in patients with ovarian carcinomas should not be ignored. In addition, endoscopic submucosal dissection (ESD) with enucleation might be a viable option. In our case, however, ESD with enucleation was not an option owing to the presence of metastases in multiple lymph nodes. Kang et al.3 reported a case of primary ovarian carcinoma with gastric metastasis that mimicked gastrointestinal stromal tumor (GIST), which was diagnosed after the patient underwent gastric antrectomy. Similarly, in the present case, we suspected that the gastric lesion was most likely GIST. On the basis of the patients medical history, we considered the possibility of either breast cancer or ovarian cancer metastasis.
Our initial diagnosis of the submucosal gastric lesion was confirmed to be gastric metastasis from ovarian cancer. The best long-term survival strategy for advanced ovarian cancer is primary debulking surgery.8 According to the National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer, secondary cytoreductive surgery can be considered for patients with recurring cancer after a long disease-free interval (6 months or more).9 While in our case, the patient's cancer recurred twice, and her last disease-free interval was more than 6 months. Therefore, gastric resection with lymphadenectomy would be the optimal treatment strategy. EUS with fine needle aspiration or biopsy is useful for the correct diagnosis of gastric submucosal lesions.7 In our case, EUS-guided fine-needle aspiration or biopsy would have ideally been applied prior to surgery. Additionally, as the patient had multiple primary cancers (thyroid cancer, breast cancer, and ovarian cancer), a further genetic analysis to detect BRCA1 or 2 mutations might be indicated.
In conclusion, although rare, a diagnosis of gastric metastasis from ovarian cancer should be considered in patients with a submucosal
gastric tumor and a history of ovarian cancer.
Figures and Tables
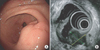 | Fig. 1Gastroduodenoscopy findings. (A) The gastroduodenoscopy showed a 2 cm submucosal tumor with erosion on the greater curvature of the antrum. (B) The mass was a heterogeneous low-echogenic lesion showing irregularity at the propria muscle layer. |
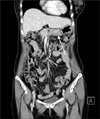 | Fig. 2Computed tomography (CT) findings. A CT scan indicated focal thickening and mass formation in the greater curvature of the gastric body (arrow) as well as multiple enlarged lymph nodes along the greater curvature of the stomach. |
 | Fig. 3Gross findings (A) and cut sections (B). A 3.5 × 3.0 cm tumor was situated over the submucosa and muscularis propria in the stomach. The mucosa of the tumor was intact. |
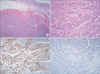 | Fig. 4Microscopic findings. (A, B) Microscopically, infiltration of metastatic serous adenocarcinoma cells into normal gastric tissues was observed. (A: H&E, ×40; B: H&E, ×200). (C) The tumor cells' immunohistochemical staining value was WT-1 positive (×200). (D) The tumor cells' immunohistochemical staining value was GCDPF-15 negative (×200). |
References
1. Zhou JJ, Miao XY. Gastric metastasis from ovarian carcinoma: a case report and literature review. World J Gastroenterol. 2012; 18:6341–6344.

2. Jung HJ, Lee HY, Kim BW, Jung SM, Kim HG, Ji JS, et al. Gastric metastasis from ovarian adenocarcinoma presenting as a submucosal tumor without ulceration. Gut Liver. 2009; 3:211–214.

3. Kang WD, Kim CH, Cho MK, Kim JW, Lee JS, Ryu SY, et al. Primary epithelial ovarian carcinoma with gastric metastasis mimic gastrointestinal stromal tumor. Cancer Res Treat. 2008; 40:93–96.

4. Kim EM, Lee BS, Moon HS, Sung JK, Kim SH, Lee HY, et al. Distal cholangiocarcinoma with gastric metastasis mimicking early gastric cancer. Gut Liver. 2009; 3:222–225.

5. Kim YI, Kang BC, Sung SH. Surgically resected gastric metastasis of pulmonary squamous cell carcinoma. World J Gastrointest Surg. 2013; 5:278–281.

7. Akce M, Bihlmeyer S, Catanzaro A. Multiple gastric metastases from ovarian carcinoma diagnosed by endoscopic ultrasound with fine needle aspiration. Case Rep Gastrointest Med. 2012; 2012:610527.

8. Schorge JO, Eisenhauer EE, Chi DS. Current surgical management of ovarian cancer. Hematol Oncol Clin North Am. 2012; 26:93–109.

9. National Comprehensive Cancer Network (NCCN). Ovarian cancer (including fallopian tube cancer and primary peritoneal cancer) version 2 [Internet]. Fort Washington (PA): NCCN;2014. cited 2014 Apr 1. Available from: http://www. nccn.org.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download