Abstract
Purpose
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been shown to improve survival in select patients with gastric cancer and peritoneal metastases. It remains unclear, however, whether this multimodal treatment protocol is also beneficial for signet-ring cell gastric cancer (SRC) patients with peritoneal metastases.
Materials and Methods
Clinical data of patients scheduled for upfront systemic chemotherapy consisting of 5-FU (2,600 mg/m2), folinic acid (200 mg/m2), docetaxel (50 mg/m2), and oxaliplatin (85 mg/m2) followed by CRS and HIPEC using cisplatin (50 mg/m2) at the Comprehensive Cancer Center, University Hospital Tübingen, Germany were retrospectively analyzed.
Results
Eighteen consecutive patients for whom irresectability has been ruled out by a computed tomography scan were enrolled. However, complete cytoreduction could only be achieved in 72% of patients. When categorizing patients with respect to the completeness of cytoreduction, we found no difference between both groups considering tumor- or patient-related factors. The overall complication rate following complete cytoreduction and HIPEC was 46%. Within a median follow-up of 6.6 (0.5~31) months, the median survival for CRS and HIPEC patients was 8.9 months as opposed to 1.1 months for patients where complete cytoreduction could not be achieved. Following complete cytoreduction and HIPEC, progression-free survival was 6.2 months.
Conclusions
In SRC with peritoneal metastases, the prognosis appears to remain poor irrespective of complete CRS and HIPEC. Moreover, complete cytoreduction could not be achieved in a considerable percentage of patients. In SRC, CRS and HIPEC should be restricted to highly selective patients in order to avoid exploratory laparotomy.
In early stage (T1 and T2) gastric cancer, surgical resection represents definitive treatment, with 5-year survival rates ranging 70% to 95%.1,2 In locally advanced tumors, however, prognosis is poor despite curative resection and extended lymphadenectomy.1,2 As a consequence, multimodal treatment strategies that consist of pre- and postoperative chemotherapy and aim for enhanced local control and improved survival have been established.
Peritoneal metastases in gastric cancer are considered to indicate terminal disease. Therapy is mainly based on palliative chemotherapy with poor long-term survival because systemic chemotherapy is unlikely to accumulate in peritoneal nodules in cytotoxic concentrations.3,4,5,6,7,8
Cytoreductive surgery (CRS) along with hyperthermic intraperitoneal chemotherapy (HIPEC) has been suggested to improve survival in select patients with limited peritoneal spread, resulting in a median overall survival (OS) of 8 to 14 months.8,9,10 It remains unclear, however, whether preoperative chemotherapy might be able to add additional oncological benefits without disproportionately raising adverse events. So far, only few clinical studies evaluated this particular treatment protocol.8,9,10 In particular, data on the management of signet-ring cell cancers with peritoneal metastases are still lacking in the current literature.
Signet-ring cell gastric cancer (SRC) is associated with poor outcome and its response to systemic chemotherapy is low. To date, it remains unclear why SRC patients tend to primarily experience peritoneal tumor spread and why their response to systemic chemotherapy is usually poor.11,12 On the basis of the low response rates to systemic chemotherapy and the lack of treatment alternatives, radical surgery with HIPEC is discussed with each patient individually as a personalized approach.
With this retrospective analysis, we sought to investigate whether preoperative chemotherapy followed by CRS and HIPEC could also be performed in SRC patients with peritoneal metastases with acceptable morbidity and mortality.
Between July 2008 and February 2013, 18 consecutive patients with histopathologically proven SRC and synchronous peritoneal metastases were enrolled in this study at the Peritoneal Surface Malignancy Program at the University of Tübingen, Germany. All patients were treated with upfront chemotherapy, followed by CRS and HIPEC. Preoperative diagnostics consisted of a thorough clinical examination, blood tests, and a computed tomography (CT) scan to rule out distant metastases. CT images were acquired by 128-slice multi-detector spiral CT at the Department of Radiology, University Hospital Tübingen, Germany. The reconstructed slice thickness was 5 mm without gaps between slices. Local irresectability was defined as the infiltration of the mesenteric axis, retroperitoneal plane, or the pancreatic head. The eligibility for CRS and HIPEC was assessed by a surgical oncologist, a medical oncologist, a radiologist, a radio-oncologist, and a clinical pathologist, who all attend a weekly interdisciplinary oncologic team meeting and present the patients' demographics and imaging results. Patients were followed in 3-monthly intervals with clinical examination and radiological imaging including CT- or positron emission tomography (PET)-CT scans. Recurrence was defined as any new lesion detected by CT or PET-CT scans compared to the findings of the first examination after CRS and HIPEC. Adverse events were classified according to the Clavien-Dindo complication score.13 Grade 1 was defined as any deviation from the normal postoperative course, and grade 2 indicated pharmacological treatment. For grade 3 complications, there was a need for radiological, endoscopic, or surgical intervention. Life threatening complications were classified as grade 4 and death as grade 5. In-hospital mortality was defined as death within 30 days of surgery. Tumors were classified by histology according to the World Health Organization classification.
Data were collected prospectively during daily routine and analyzed retrospectively. Patients were retrospectively categorized with respect to the completeness of cytoreduction. This study was performed in accordance to the local ethical guidelines.
The neoadjuvant chemotherapy protocol consisted of 4 to 6 cycles of the following regimen: 2,600 mg/m2 of 5-FU for 24 hours, 200 mg/m2 of folinic acid for 1 hour, 50 mg/m2 of docetaxel for 2 hours, and 85 mg/m2 of oxaliplatin for 2 hours.
After laparotomy through a mid-line incision and complete adhesiolysis, the peritoneal carcinomatosis index (PCI) was determined following the criteria described by Jacquet and Sugarbaker.14 Abdominal regions were categorized as the small bowel, consisting of Sugarbaker's abdominopelvic regions (SAPR) 9 to 12; the upper abdomen, consisting of SAPR 0 to 3; and the lower abdomen/pelvis consisting of SAPR 4 to 8. Then, after meticulous exploration of the small bowel, CRS was performed by gastrectomy with D2-lymphadenectomy and Roux-Y-reconstruction, along with resection of any involved adjacent structures and peritonectomy procedures described by Sugarbaker15,16,17 aiming for complete cytoreduction (CC-0 and CC-1 [CC-0 indicates no visible disease; CC-1 indicates nodules smaller than 0.25 cm]).
After complete cytoreduction and fashioning of intestinal anastomoses, HIPEC with 50 mg/m2 of cisplatin was administered for 90 minutes at 42℃ using the open coliseum-technique. If complete cytoreduction and HIPEC was achieved, patients did not receive further postoperative systemic chemotherapy.
Data are presented as median (minimum~maximum) or number (%) unless otherwise stated. Qualitative differences were compared using the χ2-test and quantitative differences were assessed using the Mann-Whitney U test. Survival analysis was performed by the Kaplan-Meier method. For OS, the time to the event was calculated as the time from CRS until death or time to last contact, if the patient was alive. Recurrence was calculated from the date of surgery to the time of relapse, or to the last known date of follow-up evaluation, or the date of death using the Kaplan-Meier method. A P-value of less than 0.05 was considered significant. SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Eighteen patients for whom there was radiographical evidence of peritoneal disease without signs of irresectability or distant metastases were scheduled for upfront chemotherapy. Intraoperatively, complete cytoreduction (CC-0 or CC-1) could be achieved in 13 patients (72%), whereas 5 patients (28%) underwent only explorative laparotomy due to either the involvement of the pancreas, or the retraction of the mesenteric axis, or tumor involvement of the small bowel surface. In these particular patients, we found both a significantly higher extent of small bowel involvement as well as a trend towards a more locally advanced tumor growth (Table 1). In one patient, a palliative gastrectomy was performed due to symptomatic gastric outlet obstruction.
Baseline demographic and intraoperative characteristics are shown in Table 2. There was no difference with respect to tumor- or patient-related factors between the 2 groups except for the intraoperative PCI (12 [1~22] vs. 37 [13~39]; P=0.003) and time in the operating room (520 [398~694] vs. 96 [60~306] minutes; P<0.0001).
In order to achieve complete cytoreduction, 7 patients (54%) underwent right upper quadrant, 5 patients (38%) left upper quadrant, and 5 patients (38%) pelvic peritonectomy. Additionally, 21 visceral resections were performed. In 46% of patients, however, proximal and/or distal gastric resection margins were histologically positive for tumor involvement (R1-resection) despite complete cytoreduction (Table 3).
The overall complication rate following complete cytoreduction and HIPEC was 46%. There were 11 adverse events (Clavien-Dindo I~IV) in 6 patients. However, there was no anastomotic leakage and no need for any re-operation. In addition, there was no inhospital death. Four patients (31%) experienced mild (<3,500/µl) temporary HIPEC-related leucopenia. Adverse events are listed in Table 4.
The median follow-up was 6.6 (0.5~31) months. Within the follow-up period, 6 patients (46%) who underwent complete cyto-reduction and HIPEC and 2 patients (40%) who underwent explorative laparotomy died. The median OS was 8.9 months following complete cytoreduction and HIPEC and 1.1 months following explorative laparotomy (Fig. 1).
Following complete CRS and HIPEC, progression-free survival was 6.2 months (Fig. 2). In 2 patients, the recurrence was located in the parietal peritoneum. One patient experienced a lymph node recurrence. Distant metastases were not observed within the follow-up period.
In our investigation, the incidence of postoperative complications was comparable with available Phase II and III studies.7,8,9,10,18 Yang et al.18 reported severe adverse events in 14.7% of patients, and Glehen et al.8 found major complications in 27.8% of patients. In their case series, Scaringi et al.7 reported that 10 out of 37 patients developed at least one complication whereas Hultman et al.9 described grade II~IV adverse events in 62.5% of patients. Our surgery-related morbidity seemed acceptable without any in-hospital deaths, even though several peritonectomy procedures and visceral resections had to be performed per patient to achieve complete cytoreduction. We observed no anastomotic leakage or duodenal fistula and no re-operations were required. The majority of adverse events were HIPEC-related, such as leucopenia. However, only mild leucopenia was found without any need for granulocyte-colony stimulating factors.
Complete cytoreduction could be achieved in 72% patients. This is in accordance with Yonemura et al.5 and Hultman et al.,10 who reported a 71% and 44% rate of complete cytoreduction, respectively.9 However, in 28% of patients radical surgery was not possible either because of the extent local growth or because of the extent peritoneal metastases on the small bowel surface, even though there was no a priori evidence of irresectability. Moreover, proximal, distal, and/or circumferential gastric resection margins were histologically proven positive in 46% of patients in whom complete cytoreduction could be achieved. This might be related to the tumor biology resulting macroscopically in linitis plastica with extensive lymphangiosis.
The median OS was low (8.9 months) and 23% of patients developed peritoneal recurrence within the follow-up period, whereas no patient developed distant metastases. Additionally, taking into account that 6 patients died within the follow-up period, peritoneal relapse occurred in approximately 50% of patients. Again, this is very likely the result of an aggressive tumor biology. The short follow-up period, however, does definitely not allow final conclusions to be drawn. In particular, we are not able to issue a statement on whether or not this multimodal treatment protocol has the potential to improve survival. Because of the low number of patients, PCI does not aid in categorizing patients suitable for CRS and HIPEC, as recommended by Yonemura et al.19
As stated above, surgery had to be terminated as explorative laparotomy in 28% of patients because of tumor spread that could not be ruled out by preoperative radiological diagnostics. However, the majority of preoperative CT scans have not been performed in our hospital. Therefore, the precise initial tumor burden as well as the response to chemotherapy could not be evaluated, which is a major limitation of our investigation. Since the hospital stay did not differ between the 2 groups and the outcome was very poor, explorative laparotomy by itself seems to have a negative impact on outcome, emphasizing the need for more appropriate selection criteria. Since there was no difference between the 2 groups with respect to tumor- and patient-related factors, we will in the future use laparoscopy and histology with response evaluation in every patient prior to performing CRS and HIPEC, in order to avoid unnecessary exploratory laparotomy. Due to the synchronous peritoneal spread, laparoscopy is likely to work well because no previous extensive oncologic surgery has been performed in these particular patients. Nonetheless, a meticulous laparoscopic assessment of the entire abdominal cavity remains challenging. However, ruling out patients with tumor progression and histological non-responders should be possible.
This multimodal protocol consisting of upfront chemotherapy, CRS and HIPEC is feasible with acceptable surgical morbidity in highly selective SRC patients with peritoneal metastases. However, survival seems to remain low and complete cytoreduction is not possible in a considerable percentage of patients despite accurate preoperative radiographic diagnostics with exploratory laparotomy, leading to a worse prognosis.
In summary, even though a randomized phase-III study by Yang et al.18 suggested that CRS and HIPEC prolong survival in patients with peritoneal metastases from predominantly non-SRC, according to our data, CRS and HIPEC cannot be recommended for patients with SRC and peritoneal metastases in general. Moreover, it seems very likely that only patients with limited peritoneal spread will benefit from this multimodal approach.19 Therefore, initial staging laparoscopy might help as a selection tool for identifying patients with both high abdominal tumor load and as being unlikely to achieve complete cytoreduction.
We modified our treatment protocol utilizing staging laparoscopy in addition to CT scans in all patients with SRC and peritoneal metastases prior to neoadjuvant chemotherapy. After chemotherapy, re-laparoscopy and biopsy is performed by the same surgical team. If the abdominal tumor load remained stable or decreased, CRS and HIPEC are performed. Patients with progressive disease continue with palliative chemotherapy or best supportive care.
Figures and Tables
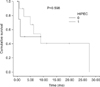 | Fig. 1Cumulative survival comparing patients following complete cytoreduction and HIPEC (HIPEC = 1) and explorative laparotomy (HIPEC = 0). HIPEC = hyperthermic intraperitonealchemotherapy. |
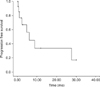 | Fig. 2Progression-free survival following complete cytoreductive surgery and hyperthermic intraperitonealchemotherapy. |
References
1. Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003; 56:1–9.

3. Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). Gastric Cancer. 2001; 4:27–33.

4. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–4997.

5. Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009; 100:311–316.

6. Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004; 139:20–26.

7. Scaringi S, Kianmanesh R, Sabate JM, Facchiano E, Jouet P, Coffin B, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008; 34:1246–1252.

8. Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010; 17:2370–2377.

9. Hultman B, Lind P, Glimelius B, Sundbom M, Nygren P, Haglund U, et al. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2013; 52:824–830.

10. Hultman B, Lundkvist J, Glimelius B, Nygren P, Mahteme H. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol. 2012; 51:112–121.

11. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009; 250:878–887.

12. Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014; 21:1739–1748.

13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
14. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374.

17. Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir. 1988; 373:189–196.

18. Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011; 18:1575–1581.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download