Abstract
Aerobic glycolysis has been the most important hypothesis in cancer metabolism. It seems to be related to increased bioenergetic and biosynthetic needs in rapidly proliferating cancer cells. To this end, F-18 fluorodeoxyglucose (FDG), a glucose analog, became widely popular for the detection of malignancies combined with positron emission tomography/computed tomography (PET/CT). Although the potential roles of FDG PET/CT in primary tumor detection are not fully established, it seems to have a limited sensitivity in detecting early gastric cancer and mainly signet ring or non-solid types of advanced gastric cancer. In evaluating lymph node metastases, the location of lymph nodes and the degree of FDG uptake in primary tumors appear to be important factors affecting the diagnostic accuracy of PET/CT. In spite of the limited sensitivity, the high specificity of PET/CT for lymph node metastases may play an important role in changing the extent of lymphadenectomy or reducing futile laparotomies. For peritoneal metastases, PET/CT seems to have a poorer sensitivity but a better specificity than CT. The roles of PET/CT in the evaluation of other distant metastases are yet to be known. Studies including primary tumors with low FDG uptake or peritoneal recurrence seem suffer from poorer diagnostic performance for the detection of recurrent gastric cancer. There are only a few reports using FDG PET/CT to predict response to neoadjuvant or adjuvant chemotherapy. A complete metabolic response seems to be predictive of more favorable prognosis.
In 1920s, Warburg et al.1 reported a phenomenon that cancer cells are dependent on glycolysis even in the presence of oxygen which is likely due to the impaired function of mitochondria. Since then, this Warburg effect has been the most important hypothesis in studying cancer metabolism and is considered as a seventh hallmark of human cancers.2 Aerobic glycolysis was originally attributable to increased bioenergetic needs in rapidly proliferating cancer cells. Recently, biosynthetic aspect of aerobic glycolysis synthesizing macromolecules such as nucleotide, fatty acid, amino acid, etc. is under active investigation. Based on this phenomenon, F-18 fluorodeoxyglucose (FDG), a glucose analog, became the most commonly used radiotracer for the detection of malignancies combined with positron emission tomography (PET)/computed tomography (CT). In this review, the potential roles of FDG PET/CT will be discussed in staging or restaging, monitoring therapeutic responses, and predicting patient clinical outcomes in gastric cancers.
Imaging modalities such as CT provides exquisite anatomic details to determine the surgical resectability of gastric cancers whereas F-18 FDG PET/CT may have roles in predicting biological aggressiveness and prognosis based on the metabolic activity of primary tumors. Until now, it has been reported that F-18 FDG PET or PET/CT has 21% to 100% of sensitivity and 78% to 100% of specificity for detecting primary tumors.3,4,5,6,7,8,9,10,11 The wide ranges of sensitivity are associated with technical and histopathological factors affecting the visibility of primary tumors on PET/CT (Fig. 1). There can be significant amounts of physiologic FDG uptake in the stomach which mimics pathology. Simple distention of the stomach using water reduces physiologic uptake and improves the diagnostic performance of PET/CT in detecting and localizing primary tumors, and assessing the degree of FDG uptake in gastric cancers.12,13,14 Other than technical issues, there are histopathological factors affecting the PET/CT visibility of gastric cancers. The tumor size is important especially for early gastric cancer (EGC) since FDG uptake is underestimated due to a partial volume averaging effect on PET/CT. Low FDG uptake is more often seen in signet ring cell and mucinous types of gastric cancer. Of the macroscopic types, Borrmann's type I has significantly higher FDG uptake than do the other 3 types. Borrmann's type 4 seems to have the least FDG uptake in primary tumors. Of the microscopic types, intestinal type has more FDG uptake than diffuse type. Regarding the differentiation, low FDG uptake was reported in poorly differentiated types, which is likely due to the low concentration of cancer cells in primary lesions.15 However, a wide spectrum of FDG uptake from low to intense is also seen in poorly differentiated adenocarcinomas. Other factors besides histologic differentiation seem important in determining FDG uptake in adenocarcinomas of poorly differentiated type.5 Overall, PET/CT has a limited sensitivity in detecting EGC and some types of advanced gastric cancer (AGC) as discussed above.
One of the benefits on PET/CT is in the prediction of biological aggressiveness and/or patient prognosis on the basis of the metabolic activity of primary tumors. There are controversial, limited data whether the degree of FDG uptake on PET/CT is predictive of patient prognosis.3,16,17,18,19,20 Some reported a longer survival in patients with negative PET than those with positive PET whereas others could not find any difference in survival rate between patients with high FDG uptake and those with low FDG uptake. Studies by histopathological subtypes seem to give better information on the association between FDG uptake and patient prognosis.18,20 In our study assessing 41 patients with curative gastrectomy for advanced signet ring cell carcinoma, with a cutoff standardized uptake value (SUV) of 3.8, the high SUV group showed more aggressive tumor behavior than did the low SUV group.18 The high SUV group also had more postoperative recurrence, shorter relapse free survival, and lower 30 months cancer specific survival rates although SUV was not an independent predictor of overall survival.
Accurate staging is essential in selecting optimal management plan for the patients preoperatively. The role of PET/CT is limited in T staging of primary tumors due to its low spatial resolution preventing the evaluation of adjacent organ invasion. The presence of lymph node metastases is one of the most important prognostic factors in gastric cancer. N staging has been typically dependent on the size of lymph node on CT. However, the size criterion is insufficient to guide the optimal extent of lymphadenectomy. There are several papers showing limited sensitivity of FDG PET or PET/CT in evaluating lymph node metastases in gastric cancer.5,7,11,21 The location of the lymph nodes and FDG uptake in primary tumors appear to have some impact on diagnostic performance of PET/CT. Despite the high specificity, it is less sensitive than CT for detecting perigastric lymph nodes. The spatial resolution of PET/CT may not be good enough to discriminate those perigastric lymph nodes from adjacent primary tumors. However, accurate staging of perigastric lymph node metastases may not be important since all AGC patients are to undergo at least D1 dissection. Unlike perigastric lymph nodes, the determination of N2 or N3 group is of clinical importance, as the extent of lymph node dissection or curative potential of surgery can be changed. So far, PET/CT show limited sensitivity of less than 50% in detecting metastases in N2 or N3 group whereas it is highly specific (over 90% or higher) for N2 or N3 node metastases. Given the high specificity for N2 or N3 disease, PET/CT may play an important role in extending the degree of lymphadenectomy or reducing futile laparotomies. For N staging, PET/CT is considered to have similar diagnostic performance to contrast enhanced CT.11 Further studies are needed to evaluate additional benefit of PET/CT in detecting small lymph nodes in which CT cannot determine the presence of metastases.
Although there are not enough data yet, FDG PET seems limited in detecting peritoneal metastases.6,15 Lim et al.22 retrospectively compared FDG PET to contrast enhanced CT in 112 patients with histological confirmation for the absence or presence of peritoneal metastases. PET showed a poorer sensitivity of 35% but a better specificity of 99% than CT which had a sensitivity of 77% and a specificity of 92%. Studies are needed to see whether the degree of FDG uptake in primary tumors may affect the sensitivity of PET/CT in detecting peritoneal metastases (Fig. 2). For the evaluation of other distant metastases including the liver, bone, lung, adrenal gland, or etc, the roles of PET/CT are yet to be known. A recent study reported that PET/CT detects occult metastases in about 10% of patients with AGC.23 They suggested PET/CT to be a component of the standard staging algorithm for AGC due to reduced morbidity from fewer futile operations and lower patient care costs.
Common locations of recurrence after initial surgery include locoregional areas, peritoneum, extra-abdominal lymph nodes, and hematogenous spread to distant sites. Although contrast CT is most commonly used in detecting recurrence, its accuracy can be compromised by anatomical alterations related to postoperative changes. PET/CT has shown inconsistent results in detecting recurrent gastric cancers.15,16,24,25,26,27,28,29,30 Primary tumors included in a study population and location of recurrent tumors might have something to do with the contradictory results of PET/CT. Studies including more primary tumors with low FDG uptake or peritoneal recurrence seem to suffer from poorer diagnostic performance.16,29 In recent data comparing PET/CT with contrast CT, PET/CT was at least as sensitive and specific as contrast enhanced CT in the detection of recurrent gastric cancers except for peritoneal metastases.28,30 Gastric distention using water is encouraged to improve the accuracy of PET in differentiating recurrent tumor from physiologic uptake in the remnant stomach although it is limited in the detection of those recurrent tumors with low FDG uptake.12
Neoadjuvant therapy has been increasingly used to reduce tumor stage, to plan the best surgical strategies, to test in vivo chemosensitivity, and to improve overall survival in patients with various locally advanced cancers. Only those patients with a clinical and pathological response to neoadjuvant therapy may have a significant survival benefit. Therefore, the early identification of responders from non-responders using clinical and/or imaging predictors seems to be essential. On CT, treatment response may be underestimated by treatment related changes such as fibrosis, necrosis, inflammation, and edema. Because changes in glucose metabolism can precede changes in morphology, FDG can be an early and sensitive pharmacodynamic marker of tumor response to treatment. It largely represents viable tumor cell number and a reduction in FDG uptake may reflect the tumor cell killing rate. Other than FDG uptake remained after the completion of treatment, changes in FDG uptake soon after the initiation of treatment is also related to final patient outcomes (Fig. 3). The latter can provide earlier assessment of treatment response in clinical trials as well as patient management.
For response evaluation, the results of FDG PET or PET/CT need to be correlated with tumor regression grades on histopathologic specimen, tumor recurrence, or patient survival. In gastric cancer, there are only a few studies using PET to predict responses to neoadjuvant or adjuvant chemotherapy. Good correlations were reported between changes in FDG uptake early during the course of neoadjuvant chemotherapy and histopathological responses.31,32 A complete metabolic response on FDG PET seems to be predictive of more favorable prognosis. In adjuvant setting, some reported that either early metabolic response or lower primary tumor SUV can predict better clinical outcome in patients with AGC.17,33
One of the limitations of FDG PET or PET/CT for response evaluation to neoadjuvant therapy is that FDG PET is not able to differentiate complete tumor response from microscopic residual tumor. Therefore, the decision on the extent of surgery cannot be made by FDG PET/CT alone. Of gastric cancers, certain histologic types may not show increased FDG which could make response evaluation difficult. Physiologic FDG uptake in the stomach without distention may underestimate tumor response to treatment. Besides these limitations, the best time to do posttreatment FDG PET/CT is still a matter of debate. Early assessment at mid-treatment seems to be useful in selecting responders from non-responders, modifying subsequent treatment, and avoiding unnecessary side effects in non-responders. Lastly, the methodology in analyzing FDG uptake or changes in SUVs is yet to be standardized.
FDG PET/CT has a limited sensitivity in detecting EGC and mainly signet ring or non-solid types of AGC. Although it does not provide exquisite anatomic details to determine the surgical resectability, it has potential roles in predicting biological aggressiveness and prognosis based on the metabolic activity of primary tumors. So far, there are controversial, limited data whether the degree of FDG uptake on PET/CT is predictive of patient prognosis. FDG PET/CT has limited sensitivity in evaluating lymph node metastases. The location of lymph nodes and FDG uptake in primary tumors appear to be important factors affecting the diagnostic accuracy of PET/CT. Given the high specificity of PET/CT for lymph node metastases, it may play an important role in changing the extent of lymphadenectomy or reducing futile laparotomies. For the detection of peritoneal metastases, PET/CT seems to have a poorer sensitivity but a better specificity than CT. For the evaluation of other distant metastases, the roles of PET/CT are yet to be known. PET/CT has shown inconsistent results in detecting recurrent gastric cancers. Studies including more primary tumors with low FDG uptake or peritoneal recurrence seem suffer from poorer diagnostic performance. There are only a few reports using FDG PET to predict response to neoadjuvant or adjuvant chemotherapy. A complete metabolic response on FDG PET seems to be predictive of more favorable prognosis.
Figures and Tables
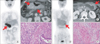 | Fig. 1Histopathologic factors affecting fluorodeoxyglucose (FDG) uptake in advanced gastric cancers (AGCs). (A, B, C) AGC with intense FDG uptake (arrows) and intestinal growth pattern (H&E, ×100). (D, E, F) AGC with mild FDG uptake (arrows) and diffuse growth pattern (H&E, ×100). |
 | Fig. 2Peritoneal seeding metastases with variable F-18 fluorodeoxyglucose (FDG) uptake on positron emission tomography/computed tomography. (A) Typical metastatic nodules with high FDG uptake (arrows) in the omentum. (B) Mild and diffuse FDG uptake (arrows) along the omentum. |
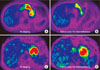 | Fig. 3Changes in fluorodeoxyglucose (FDG) uptake of the primary tumors on positron emission tomography/computed tomography (PET/CT) to neoadjuvant chemotherapy. (A, B) PET responder, FDG uptake in the primary tumor showing more than 35% decreases in standardized uptake value (SUV) which is predictive of histopathological response and better patient survival. (C, D) PET non-responder, no remarkable changes in SUV in the primary tumor. |
References
1. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927; 8:519–530.

3. Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003; 30:288–295.

4. Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004; 28:247–253.

5. Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005; 46:1582–1588.
6. Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005; 103:2383–2390.

7. Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155.

8. Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006; 9:192–196.

9. Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007; 48:1945–1950.

10. Kameyama R, Yamamoto Y, Izuishi K, Takebayashi R, Hagiike M, Murota M, et al. Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2009; 36:382–388.

11. Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011; 79:183–188.

12. Yun M, Choi HS, Yoo E, Bong JK, Ryu YH, Lee JD. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET. J Nucl Med. 2005; 46:953–957.
13. Kamimura K, Nagamachi S, Wakamatsu H, Fujita S, Nishii R, Umemura Y, et al. Role of gastric distention with additional water in differentiating locally advanced gastric carcinomas from physiological uptake in the stomach on 18F-fluoro-2-deoxy-D-glucose PET. Nucl Med Commun. 2009; 30:431–439.

14. Takahashi H, Ukawa K, Ohkawa N, Kato K, Hayashi Y, Yoshimoto K, et al. Significance of (18)F-2-deoxy-2-fluoro-glucose accumulation in the stomach on positron emission tomography. Ann Nucl Med. 2009; 23:391–397.

15. Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003; 44:690–699.
16. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002; 29:525–529.

17. Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010; 136:1929–1935.

18. Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol. 2011; 104:566–570.

19. Park JC, Lee JH, Cheoi K, Chung H, Yun MJ, Lee H, et al. Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: the maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging. 2012; 39:1107–1116.

20. Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012; 39:1425–1434.

21. Yang QM, Kawamura T, Itoh H, Bando E, Nemoto M, Akamoto S, et al. Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology. 2008; 55:782–785.
22. Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006; 7:249–256.

23. Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG, et al. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012; 118:5481–5488.

24. Jadvar H, Tatlidil R, Garcia AA, Conti PS. Evaluation of recurrent gastric malignancy with [F-18]-FDG positron emission tomography. Clin Radiol. 2003; 58:215–221.

25. Nakamoto Y, Togashi K, Kaneta T, Fukuda H, Nakajima K, Kitajima K, et al. Clinical value of whole-body FDG-PET for recurrent gastric cancer: a multicenter study. Jpn J Clin Oncol. 2009; 39:297–302.

26. Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009; 34:441–447.

27. Sohn YJ, Jang JS, Choi SR, Kwon HC, Jung GJ, Kim MC, et al. Early detection of recurrence after endoscopic treatment for early gastric cancer. Scand J Gastroenterol. 2009; 44:1109–1114.

28. Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009; 9:73.

29. Lee JE, Hong SP, Ahn DH, Jeon TJ, Kang MK, Kwon CI, et al. The role of 18F-FDG PET/CT in the evaluation of gastric cancer recurrence after curative gastrectomy. Yonsei Med J. 2011; 52:81–88.

30. Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients' treatment decision making? Eur J Nucl Med Mol Imaging. 2011; 38:64–73.

31. Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003; 21:4604–4610.

32. Ott K, Herrmann K, Lordick F, Wieder H, Weber WA, Becker K, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res. 2008; 14:2012–2018.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download