Abstract
Purpose
The intracorporeal reconstruction after laparoscopic gastrectomy can minimize postoperative pain, and give better cosmetic effect, while it may have technical difficulties and require the learning curve. This study aimed to analyze the surgical outcome of intracorporeal reconstruction according to the surgeon's experience comparing with extracorporeal procedure.
Materials and Methods
From January 2009 to September 2011, intracorporeal reconstruction in laparoscopic surgery for gastric cancer was performed for 71 patients (Intra group). During same period, 231 patients underwent laparoscopy-assisted gastrectomy (Extra group). These patients were classified into initial (1st to 20th case of intra group), intermediate (21th to 46th case), and experienced (after 47th case) phases.
Results
Intracorporeal procedures included 35 cases of Billroth-I, 30 Billroth-II and 6 Roux en Y reconstructions. In the initial phase, operation time (P=0.022) were significantly longer for the patients of intra group than them of extra group. Although the difference was not significant, the length of hospital stay was longer and complication rate was higher in the intra group. In intermediate and experienced phases, there was no difference between two groups in operation time and hospital stay. In these phases, complication rate was lower in the intra group than the extra group (3.9% versus 9.7%). The pain scale was significantly lower post operation day 5 in the intra group.
The frequency of diagnosis of early gastric cancer has been recently increasing due to external influences such as increased need for health examination. Accordingly, as an interest in minimally invasive surgery considering the quality of life of patients with early gastric cancer increases, its need is also rapidly increasing. Since Kitano et al.1 reported laparoscopy-assisted gastrectomy in 1994, laparoscopy-assisted gastrectomy has become a common treatment option for early gastric cancer due to advantages such as less invasiveness, less pain, and short hospitalization. In addition, as many recent studies reported that the oncological result of laparoscopy-assisted gastrectomy was similar to that of open gastrectomy,2-5 laparoscopy-assisted gastrectomy has been more widely used, and has become a standard for the treatment of gastric cancer.
Laparoscopy-assisted gastrectomy, however, has a disadvantage of requiring small incision, which causes various problems during gastric anastomosis via a small incision. In particular, critical problems have been observed in patients with obesity.2,3 Thus, totally laparoscopic distal subtotal gastrectomy and intracorporeal anastomosis have been introduced as less invasive and more useful techniques with advantageous eye-field securing and applying less stress to the tissues in order to overcome the afore-mentioned problems of laparoscopy-assisted gastrectomy.6,7 As intracorporeal anastomosis is associated with disadvantages of technical difficulty and longer time of anastomosis, the technique demands the presence of experienced surgeons to conduct the surgery safely.8,9 Nevertheless, considering the advantages of totally laparoscopic subtotal gastrectomy and intracorporeal anastomosis, they are expected to sufficiently contribute in improving patient's quality of life, if surgeons are well trained.
Accordingly, this study was conducted to investigate the learning curve required by surgeons to conduct successful intracorporeal anastomosis following totally laparoscopic subtotal gastrectomy, and to investigate the clinical usefulness of laparoscopic distal subtotal gastrectomy by comparing its clinical outcomes with those of extracorporeal anastomosis.
This study was conducted on 71 patients who underwent intracorporeal anastomosis following totally laparoscopic distal subtotal gastrectomy after diagnosed with gastric adenocarcinoma of the mid and lower stomach in the Department of Surgery at Ajou University for a period of 33 months from January 2009 to September 2011. The medical records of the subjects were retrospectively compared with those of 230 patients who underwent extracorporeal anastomosis following laparoscopic distal subtotal gastrectomy during the same period. The subjects were divided into the early, mid, and late groups according to the frequency and surgery period of totally laparoscopic distal subtotal gastrectomy and then compared with patients with extracorporeal anastomosis. In addition, operation time, postoperative complications, and postoperative pain were also reviewed. All the subjects, who participated in the study were the ones diagnosed with T2NI or less in preoperative endoscopy, abdominal computed tomography, and endoscopic ultrasonography, and had tumor positioned at the mid 1/2 or lower, which enabled subtotal gastrectomy after resection. The proximal area of the tumor was marked with a clip during preoperative endoscopy to determine gastric resection site, and abdominal X-ray following foaming agent intake was conducted to confirm lesion site marked with a clip (Fig. 1). During the surgery, the lesser curvature and greater curvature of the mid body were marked with a clip, and subsequently abdominal X-ray was conducted to determine resection site that was sufficiently apart from the preoperative marked lesion, followed by gastric resection.
In the case of the intracorporeal anastomosis group, the stomach was pulled out from the abdomen through the infraumbilical wound immediately after resection of the stomach to assess whether the location from the resection plane to the lesion was appropriate. In the case of the extracorporeal anastomosis group, the stomach was taken out through the small incision without resection of the stomach and was then resected after confirming the gastric resection margin.
The resection range of the lymph node (LN) of D1+β or more was basically employed, but the resection range of the LN decreased to D1+α if tumor restricted to the mucosa was found in patients with concurrent diseases or elderly patients via endoscopic ultrasonography.
The patients were on a supine position under general anesthesia, and the surgeon, and the first and second assistants were positioned on the right and left side of the patients, respectively. In some cases, the second assistant who controlled a camera was positioned on the right of the patients, that is, below the surgeon. The patients had their head lifted approximately 15°. A 10 mm trocar for camera was inserted below umbilicus while maintaining a abdominal pressure of 12~13 mmHg, and a 12 mm trocar was inserted into the left and right lower abdomen (one for each area), and a 5 mm trocar was inserted into the left and right upper abdomen (one for each area). The liver was moved towards the upper abdominal wall using prolene suture to secure visual field. This surgical technique has been previously introduced by the author's hospital.10
After dissecting from the center of greater omentum towards the left direction to the anticipated resection site of the greater curvature, the branch of the left gastroepiploic vessels, which heads for the spleen, was separated and then ligated to resect 4sb LN. Subsequently, after dissecting from the center of greater omentum towards the right direction, followed by resection, the right gastroepiploic vessels were ligated at the origin to resect No.6 LN.
During D2 lymphadenectomy, the resection was performed towards the direction of the inferior border of the pancreatic head, and then the superior mesenteric vein was exposed, followed by resecting 14v LN. The right gastric artery was ligated at the origin to resect the 5 and 12a LN, and the duodenum was resected using a 60 mm laparoscopic linear stapler. The lesser omentum was resected to the left direction, followed by resecting 7, 8a, and 9 LNs. The left gastric artery was ligated at the origin, followed by resecting 11p LN.
After confirming via abdominal X-ray, the site was resected using two linear staplers, and then put into a pouch, followed by pulling it by extending the vertical incision line of the infraumbilical wound of size approximately 2 cm (Fig. 2). As mentioned above, in the case of the extracorporeal anastomosis group, the stomach was pulled out through the small incision without resecting the stomach, and then the gastric resection margin was confirmed via stomach resection. In the case of the intracorporeal anastomosis group, the sufficiency of the resection margin of the resected tissue was examined with eyes before anastomosis, and mandatorily confirmed via confirmative frozen section biopsy to secure the stability of the resection margin before anastomosis.
Intracorporeal gastroduodenostomy was conducted according to the method previously presented.11 In brief, one hole was made at the upper edge angle of the duodenal resection margin, and the another hole was made at the greater curvature approximately 7 cm upper than the gastric resection margin so as not to overlap the gastric section plane and gastroduodenal site and to secure a space for stapler. After both the blades of the linear stapler were inserted into the two holes, gastroduodenostomy was prepared at the greater curvature of the stomach and the posterior upper edge of the duodenum (Fig. 3A). After grabbing the gastroduodenal anastomotic area and both the edges using three or more laparoscopic sutures in coordination with the first assistant to position them vertical to the cartridge of gastroduodenostomy, the open hole was sutured by shooting one linear stapler to position the finished anastomosis parallel to gastroduodenostomy. In some cases, the anastomotic edge was supplemented via supplementary suture with a suture or metal clip to prevent anastomotic leakage due to tension.
Intracorporeal gastrojejunostomy was conducted instead of gastroduodenostomy in the case where the tension of the anastomized area might be caused due to relatively higher tumor location. A hole was made using a harmonic scarpel approximately 25 cm away from the Treitz ligament towards the distal area, and the other hole was made using a harmonic scarpel approximately at the greater curvature of the stomach 1.5 cm away from the gastric resection plane or the edge of the greater curvature of the gastric resection plane. After removing the gastric contents using suction, both the blades of the linear stapler were inserted into the duodenal and gastric holes, towards the distal area and spleen directions, respectively, and then the greater curvature of the stomach and the area opposite to the jejunal mesentery were anastomized using a stapler. The hole, via which the stapler was pulled out, was also closed using a linear stapler after grabbing the anastomotic area and the edge area by the first assistant, and the bleeding site was sutured using a clip or suture (Fig. 3D~F).
In the case of intracorporeal Roux-en-Y anastomosis, jejunum was divided 25 cm away from the Treitz ligament using a linear stapler. For gastrojejunostomy, a hole was made at the edge of the greater curvature of the gastric resection plane, and then the other hole was made at efferent jejunum (Fig. 3G). And a jejunojejunostomy was made 25 cm distal to the gastrojejunostomy with a linear stapler. In the cases of the other extracorporeal anastomosis groups, the anastomosis was performed via a new small incision of the upper abdomen. The anastomosis was made in similar ways as shown in the case of intraperitoneal anastomosis group or sometimes hand-sewn anastomosis was performed.
A statistical analysis was conducted using SPSS version 18.0 (IBM Co., Armonk, NY, USA). Un-paired t-test and chi-square test were conducted for analyzing continuous variables and absolute variables, respectively, in the comparison between the two groups. The continuous variables were expressed as mean±standard deviation. If P-value<0.05, it was considered statistically significant. NPIS pain scale was used for measurement of pain.
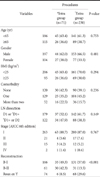
As for patient's age, 186 patients were aged 65 years or less and 115 patients were aged more than 65 years. As for body mass index (BMI), 206 patients had a BMI of 25 or less and 95 patients had a BMI of more than 25. With respect to type of surgery, the intracorporeal anastomosis group was comprised of 35 cases of B-I, 31 cases of B-II, and 6 cases of Roux-en-Y, respectively, whereas the extracorporeal anastomosis group was comprised of 131 cases of B-I, 31 cases of B-II, and 68 cases of Roux-en-Y, respectively. The rates of B-I and Roux-en-Y were higher in the extracorporeal anastomosis group than in the intracorporeal anastomosis group (Table 1).
When the two patient groups were divided into the early, mid, and late groups, and then compared with each other, no significant differences in the age, BMI, gender, the number of underlying diseases, and resection stage of LN were found between the two groups (Table 2).
As for the change in the white blood cell count was concerned, no significant difference was found between the two groups on first, second and fifth days after the surgery. However, with respect to change in pain, a significantly lower pain was observed in the intracorporeal anastomosis group on fifth day after the surgery (Fig. 4).
As for the postoperative outcomes, the bleeding amount was significantly lower in the intracorporeal anastomosis group than in the extracorporeal anastomosis group. No significant differences in the postoperative complications were found between the two groups (Table 3).
When each group was divided into the early, mid, and late groups, and then compared, the operation time was observed to be longer in the intracorporeal anastomosis group than in the extracorporeal anastomosis group for the early group, but was similar between the two groups for the mid and late groups. A significant decrease in the bleeding amount was observed in the mid and late groups. No significant difference in the hospitalization duration and complications was found between the two groups for the early group, but slight decrease was observed for the mid and late groups (Table 4). The aforementioned results demonstrated that the postoperative advantages of laparoscopic surgery12 were more highlighted in the intracorporeal anastomosis group than in the extracorporeal anastomosis group.
No case of change to open surgery or death due to the surgery was found in both the groups. One case of requiring reoperation occurred in the intracorporeal anastomosis group due to postoperative complications. Gastrojejunostomy was conducted due to anastomotic stricture of gastroduodenostomy. Other complications were resolved without any further particular treatment.
The mean length of the incision line below the bellybutton measured after the surgery was shown to be 2.7±0.3 cm, showing a satisfactory result in terms of aesthetic perspective. The pathological findings of the patients are summarized in Table 2. Two patients of the intracorporeal anastomosis group were diagnosed with disease stage III after the surgery. Since anticancer therapy termination, no reoccurrence has been reported in the two patients as of 11 and 8 months after the surgery, respectively.
Since Kim13 first reported laparoscopic surgery for patients with benign gastric diseases in Korea, laparoscopic surgery has gradually been expanding to patients with gastric cancer. As there has been an increase in interest in the improvement of the quality of life of early gastric cancer patients with high cure rate, the number of patients undergoing laparoscopy-assisted gastrectomy is also been increasing rapidly.14 Accordingly, intracorporeal anastomosis that reduces surgical injury and extracorporeal surgery have been expected to positively affect quality of life of patients, and an interest in intracorporeal anastomosis has increased in surgeons. As shown in this study, although intracorporeal anastomosis, as a reconstruction method following laparoscopy assisted distal subtotal gastrectomy, requires somewhat learning curve as compared to extracorporeal anastomosis, the technique (intracorporeal anastomosis) would not only become a safe, rapid, and easy way without any particular complication but with good outcomes, if surgeons with sufficient experience on extracorporeal anastomosis are trained approximately 20 times. This study is unique when compared to intracorporeal anastomosis with extracorporeal anastomosis following distal subtotal gastrectomy and amongst other studies on learning curve for laparoscopic surgery.15-19 Although previous studies also reported learning curve for totally laparoscopic intracorporeal anastomosis, no detailed description on the required learning curve was provided as a main subject.8,9
In this study, the bleeding amount decreased in the mid and late groups of the intracorporeal anastomosis group. From a surgical method perspective, the anatomical location and oncological view of the lesion should be considered when gastroduodenostomy is applied after subtotal gastrectomy. If lesions are progressive or close to the duodenum, it is difficult to obtain sufficient resection margin with no tension between the gastroduodenal anastomotic areas so that gastroduodenostomy cannot be used.20 In the case of no such restriction, gastroduodenostomy is preferred as a reconstruction following laparoscopic subtotal gastrectomy, most of which is conducted using circular stapler via 4~7 cm incision at the upper abdomen. If this kind of small incision is used, pulling out the stomach from the abdomen causes excessive retraction, which eventually leads to bleeding caused by tissue injury by forcing tension to the nearby tissues, as well as additional problems such as bleeding caused by rectus muscle injury or postoperative pain. Furthermore, it makes anastomosis itself problematic. In particular, in the case of high BMI, intraoperative complications, as increase in the rate of bleeding amount was reported.21 Meanwhile, in the case of intracorporeal anastomosis, it is good for securing visual field despite the difficulty in technique so that it could sufficiently overcome the aforementioned problems of extracorporeal anastomosis. In this study, no difference in the amount of bleeding was observed in the early group between the other two groups. This is likely attributable to the fact concerning slow adaptation to the use of apparatus for tissue retraction and the use of surgical stapler during the early stage. However, once the surgeons were sufficiently adapted to the technique and the required learning curve was achieved, a significant difference between the two groups was observed due to the advantages of intracorporeal anastomosis, such as no small incision, no excessive tissue retraction, and wide visual field securing.
As extracorporeal anastomosis absolutely requires small incision, strictly speaking, it is deviated from the concept of minimal invasive surgery. That is, it reduces the advantages of laparoscopic surgery as a minimal invasive surgery.6 On the contrary, intracorporeal anastomosis, as a minimal invasive surgery, can sufficiently utilize the advantages of laparoscopic surgery such as aesthetic effect, pain reduction, and reduced complication. Furthermore, intracorporeal anastomosis is advantageous with respect to that it can secure visual field regardless of patient's body type.21
Intracorporeal anastomosis requires advanced laparoscopy because of which the beginners have a difficulty in conducting intracorporeal anastomosis. In addition, as shown in this study, increased complications could lead to serious problems. Thus, intracorporeal anastomosis is recommended to surgeons, who have conducted laparoscopy-assisted extracorporeal anastomosis for 60 or more times.16-19 The corresponding author of this study has an experience on conducting approximately 500 cases of extracorporeal anastomosis. Surgeons who have sufficient experience of extracorporeal anastomosis will be able to reach quickly the learning curve for intracorporeal anastomosis.15-19 In addition, beginners could be able to conduct intracorporeal anastomosis successfully if they are sufficiently well trained. In particular, if the beginners practice starting from gastrojejunostomy, which is the easiest for the beginners, they could eventually feel confident with intracorporeal anastomosis.
In this study, the pain level significantly decreased 5 days after the surgery because of no requirement of small incision. However, as patient controlled analgesia treatment has been commonly conducted on patients with gastric cancer in the authors' hospital, it was impossible to compare the pain level due to the effect of patient controlled analgesia treatment on first and second days after the surgery, and due to insufficient data on third and fourth days after the surgery. Thus, it was possible to compare the pain level on fifth days after the surgery as the effect of painless treatment was resolved (Fig. 4).
In addition, in the short-term follow-up, a 44-year-old male patient showed postoperative persistent nausea and vomiting. Subsequently, he was diagnosed with anastomotic stricture via endoscopic examination. The result of video analysis showed that the stomach and duodenum were insufficiently inserted into the end of the linear stapler during gastroduodenostomy, and that the stapler was inserted too deep during the closing of the incision, which caused further shortening of anastomotic length. Thereafter, a particular precaution was given to secure sufficient anastomotic length. The patient with anastomotic stricture requiring an additional surgery underwent a re-surgery 4 days after the surgery, converting into gastrojejunostomy. The other patients were discharged without any complications. The result of endoscopic examination conducted in the follow-up also showed that good outcomes were observed without stricture.
Intracorporeal anastomosis is disadvantageous as it is difficult to determine the exact location of tumor since the tumor is not directly sensed by the hands during the surgery. Thus, as mentioned previously in this study, the tumor was marked with a clip via an endoscope before the surgery, and abdominal X-ray was then conducted during the surgery to secure the sufficient resection margin (Fig. 4). The resected stomach was pulled out from the abdomen and checked. If the length of the resection margin from the lesion was insufficient, resection was again conducted to secure sufficient resection margin in some cases. Medical institutions that provide totally laparoscopic surgery have been making an effort to secure sufficient resection margin using various methods.7,22 As the authors' hospital has been also developing various methods including endoscopic technique, a better technique to overcome the current disadvantages is expected to be developed in the near future, via which the disadvantages of totally laparoscopic surgery will be overcomed and intracorporeal anastomosis will become a useful technique to improve the quality of life of patients with early gastric cancer.
This study has a few limitations. Gastroduodenostomy and Roux-en-Y reconstruction were relatively more frequent in the extracorporeal anastomosis group. This is likely to be associated with a study that was conducted to compare gastroduodenostomy with Roux-en-Y reconstruction during the similar period. Based on the results of studies reporting that bile reflux was minimized, Roux-en-Y reconstruction was preferred by some institutions.23,24 However, in most of the large hospitals in Asian countries, including Korea, where the number of patients with gastric cancer is higher as compared to western countries, Roux-en-Y reconstruction seems to be less preferred than gastroduodenostomy due to longer operation time and complexity. In this study, Roux-en-Y gastrojejunostomy was not preferred in the intracorporeal anastomosis group due to the requirement of more complex techniques and more number of linear staplers.25 From this perspective, intracorporeal anastomosis has disadvantages of technical difficulty and high cost due to the requirement of more number of linear staplers during anastomosis.8 Straight line-like anastomosis used by the authors during gastroduodenostomy was relatively easily conducted as the stomach and duodenum were anastomized using a smaller hole and subsequently the hole was closed using one linear stapler, which had an advantage of reducing the number of required linear staplers. In the cases of gastrojejunostomy and Roux-en-Y gastrojejunostomy, the number of required linear staplers and leakage were reduced by making a small hole and by suturing the hole using hand-sewn suturing. In this study, hand-sewn anastomosis was performed to reduce the number of required staplers. However, although the number of the required stapler was reduced to one or two by suturing the hole using hand-sewn anastomosis, this technique was shown to require more time. Thus, an anastomotic method that not only reduces the number of staplers but also shortens operation time should be developed. In addition, the assessment and analysis of increase in cost by this technique were not performed in this study. This study was conducted by a single surgeon, hence it was difficult to generalize it. Furthermore, no long-term follow-up data are available, and the explanation of the learning curve was insufficient due to the comparison of several techniques together. The aforementioned limitations should be considered when conducting a prospective study.
In conclusion, intracorporeal anastomosis following totally laparoscopic distal subtotal gastrectomy requires an advanced performance and surgeon's learning curve. However, beginners can overcome this difficulty if they build sufficient experience. In conclusion it is proposed that intracorporeal anastomosis could become a sufficiently useful and relatively safe technique if it is repeatedly conducted for approximately 20 times. A long-term follow-up, analysis and assessment of treatment cost, and obtaining sufficient data from many institutions are further required to validate the safety and efficacy of intraperitoneal anastomosis.
Figures and Tables
Fig. 1
Localization of the location of the tumor. After clipping with endoscopy, preoperative abdomen X-ray was performed.
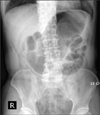
Fig. 2
Placement of surgical ports and wound for specimen delivery. For C, E, 12 mm ports are used. Two 5 mm ports are used for B, D, and flexible endoscope is introduced via port A. Port A are extended vertically for specimen delivery.
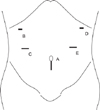
Fig. 3
Intracoporeal BI anastomosis (A, B, C), intracoporeal BII anastomosis (D, E, F), intracoporeal Roux en Y anastomosis (G, H, I). (A) Cut of duodenum upper edge. (B) Closure of endo GIA entry hole. (C) Laparoscopic view of the gastroduodenostomy. (D) Anastomosis with endo GIA. (E) Closure of endo GIA entry hole. (F) Laparoscopic view of the gastrojejunostomy. (G) Jejunojejunostomy with endo GIA. (H) Gastrojejunostomy with endo GIA. (I) Laparoscopic view of Roux en Y anastomosis.
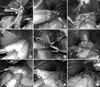
Fig. 4
Change of postoperative pain scale after sugery. Each P-value was evaluated by student T-test. POD = post operation day.

Table 2
Comparison of demographic & clinicopathologic features between intra and extra group according to learning curve

Acknowledgments
This work was supported by a grant of the Korea Healthcare Technology R&D project, Ministry of Health, Welfare, & Family Affairs, Republic of Korea (1020410).
References
1. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994. 4:146–148.
2. Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002. 131:1 Suppl. S306–S311.

3. Kim MC, Kim KH, Kim HH, Jung GJ. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. 2005. 91:90–94.

4. Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006. 12:7676–7683.

5. Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007. 245:68–72.

6. Kim HI, Woo Y, Hyung WJ. Laparoscopic distal gastrectomy with an intracorporeal gastroduodenostomy using a circular stapler. J Am Coll Surg. 2012. 214:e7–e13.

7. Kim JJ, Kim SK, Jun KH, Kang HC, Song KY, Chin HM, et al. Clinical usefulness of a totally laparoscopic gastrectomy. J Korean Gastric Cancer Assoc. 2007. 7:132–138.

8. Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009. 23:2374–2379.

9. Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008. 22:436–442.

10. Oh DK, Hur H, Kim JY, Han SU, Cho YK. V-shaped liver retraction during a laparoscopic gastrectomy for gastric cancer. J Gastric Cancer. 2010. 10:133–136.

11. Song HM, Lee SL, Hur H, Cho YK, Han SU. Linear-shaped gastroduodenostomy in totally laparoscopic distal gastrectomy. J Gastric Cancer. 2010. 10:69–74.

12. Tanimura S, Higashino M, Fukunaga Y, Kishida S, Nishikawa M, Ogata A, et al. Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc. 2005. 19:1177–1181.

13. Kim HH. Laparoscopic Billroth-II gastrectomy for benign gastric disease. Korean Soc Endosc Laparosc Surg. 1999. 2:11–18.
14. Korean Laparoscopic Gastrointestinal Surgery Study Group. Nationwide survey of laparoscopic gastric surgery in Korea, 2004. J Korean Gastric Cancer Assoc. 2005. 5:295–303.
15. Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007. 21:28–33.

16. Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005. 11:7508–7511.

17. Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2008. 18:236–241.

18. Kim KC, Yook JH, Choi JE, Cheong O, Lim JT, Oh ST, et al. The learning curve of laparoscopy-assisted distal gastrectomy (LADG) for cancer. J Korean Gastric Cancer Assoc. 2008. 8:232–236.

19. Yoo CH, Kim HO, Hwang SI, Son BH, Shin JH, Kim H. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon's learning curve period. Surg Endosc. 2009. 23:2250–2257.

20. Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg. 2008. 247:962–967.

21. Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011. 25:1395–1401.

22. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc. 2005. 19:1353–1357.

23. Chan DC, Fan YM, Lin CK, Chen CJ, Chen CY, Chao YC. Roux-en-Y reconstruction after distal gastrectomy to reduce enterogastric reflux and Helicobacter pylori infection. J Gastrointest Surg. 2007. 11:1732–1740.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download