Abstract
Purpose
The aims are to: (i) display the multidimensional learning curve of totally laparoscopic distal gastrectomy, and (ii) verify the feasibility of totally laparoscopic distal gastrectomy after learning curve completion by comparing it with laparoscopy-assisted distal gastrectomy.
Materials and Methods
From January 2005 to June 2012, 247 patients who underwent laparoscopy-assisted distal gastrectomy (n=136) and totally laparoscopic distal gastrectomy (n=111) for early gastric cancer were enrolled. Their clinicopathological characteristics and early surgical outcomes were analyzed. Analysis of the totally laparoscopic distal gastrectomy learning curve was conducted using the moving average method and the cumulative sum method on 180 patients who underwent totally laparoscopic distal gastrectomy.
Results
Our study indicated that experience with 40 and 20 totally laparoscopic distal gastrectomy cases, is required in order to achieve optimum proficiency by two surgeons. There were no remarkable differences in the clinicopathological characteristics between laparoscopy-assisted distal gastrectomy and totally laparoscopic distal gastrectomy groups. The two groups were comparable in terms of open conversion, combined resection, morbidities, reoperation rate, hospital stay and time to first flatus (P>0.05). However, totally laparoscopic distal gastrectomy had a significantly shorter mean operation time than laparoscopy-assisted distal gastrectomy (P<0.01). We also found that intra-abdominal abscess and overall complication rates were significantly higher before the learning curve than after the learning curve (P<0.05).
The use of laparoscopic gastrectomy to treat early gastric cancer has recently gained acceptance for its minimal invasiveness, making it a suitable alternative method to an open procedure.1,2 Totally laparoscopic distal gastrectomy (TLDG) including intracorporeal gastrointestinal anastomosis for gastric cancer is increasingly performed as surgeons gain experience and laparoscopic instruments continue to evolve. Reports focused on its feasibility, cosmesis, minimal invasiveness, and speedy recovery are increasing.3-5 However, some technical difficulties remain, including doubts about oncological safety (e.g., sufficient tumor-free margin) and surgeon unfamiliarity with performing intracorporeal anastomosis. For these reasons, laparoscopy-assisted distal gastrectomy (LADG) including extracorporeal anastomosis has also been a common laparoscopic surgery procedure.
Surgeons, who are currently practicing the TLDG procedure, and even experienced laparoscopic experts, should be aware that it is considered an advanced laparoscopic procedure and that it carries a significant learning curve. Although many studies have been reported on LADG learning curves, no report has yet introduced the TLDG learning curve.6-9
The aims of this study were to: (i) display the multidimensional TLDG learning curve performed by two experienced laparoscopic surgeons, (ii) verify the feasibility and effectiveness of TLDG after learning curve completion by comparing it with LADG; and (iii) compare morbidities before and after TLDG learning curve completion.
Using the Gyeongsang National University Hospital Gastric Cancer Database, an analysis of the TLDG learning curve was conducted on 180 patients (surgeon A, 121; surgeon B, 59) who underwent TLDG for the treatment of gastric cancer from July 2009 to June 2012. Three sequential variables, operation time, hospital stay, and time to first flatus, were used to define the learning curve with a moving average method. The patients were divided into 12 and 6 sequential groups of 10 patients each for the 2 surgeons, respectively.
The cumulative sum (CUSUM) method was used to investigate the outcomes of the 180 patients. The CUSUM method is useful for monitoring performance and defining a learning curve.6,10 The surgical outcome data were converted to the "success" and "failure" formats necessary for CUSUM analysis. "Success" was defined by the absence of failure points. "Failure" was defined as an open conversion, in-hospital mortality, severe complication (accordion severity classification of postoperative complications [ASCPC] score >II),11 prolonged hospital stay (>30 days), and re-admission within a month. CUSUM is defined as Sn=Σ(Xi-Xo), where Xi=0 was for success and 1 for failure.12 For this study, Xo, the "acceptable failure rate" for TLDG and its subcategories, was set at 10%. In practice, this means that for each failed TLDG, the CUSUM increased by an increment of 0.9, while each success reduced it by 0.1.
A total of 247 consecutive patients who underwent LADG (n=136) and TLDG (n=111) were included in this analysis. A total of 639 patients who underwent overall laparoscopic gastrectomy for the treatment of gastric cancer between January 2005 and June 2012 were identified. We excluded patients who the following: underwent total, proximal, or partial gastrectomy, underwent LADG and TLDG during each learning period by the 2 surgeons, and pathologic T stages >T1 to ensure homogeneity between the comparison groups.
Two experts participated in this study. By June 2012, these 2 experts had gained experience with 495 and 144 cases of laparoscopic gastrectomy, respectively. The 2 surgeons standardized all laparoscopic gastrectomy procedures and the critical postoperative management pathway.
Clinicopathological information such as age, sex, body mass index, co-morbidity, American Society of Anesthesiologists (ASA) Physical Status score, lymph node metastasis, tumor diameter, number of retrieved lymph nodes, and lengths of the proximal and distal resection margins were extracted from the database. Pathological results were classified by the 7th American Joint Committee on Cancer Staging Manual.13 Additional information on the early surgical outcome (e.g., postoperative complication, reoperation, mortality, hospital stay, and time to first flatus) and operative data (e.g., operation time, range of lymph node dissection, type of reconstruction, open conversion rate, and combined resection) were collected via electronic medical record review.
Lymph node station numbers were scored according to the Japanese classification of gastric carcinoma.14 Gastric resection and determination of the dissection area of the lymph node stations were performed based on the 2010 Japanese gastric cancer treatment guidelines.15
With regard to conventional LADG, when preoperative diagnosis using gastrofiberoscopy and spiral computed tomography (CT) scans revealed early gastric cancer, distal gastrectomy, partial omentectomy, and D1+lymph node dissection (1, 3, 4sb, 4d, 5, 6, 7, 8a, 9) were performed. For the reconstruction, a Billroth I or Billroth II procedure using a 5 cm right subcostal or upper midline mini-laparotomy was performed. When performing the Billroth I procedure, while an extracorporeal anastomosis was initially made, the intracorporeal Billroth I stapled anastomosis was created by using a hand-access device as previously reported.16 We performed antecolic and isoperistaltic gastrojejunostomy anastomosis (Billroth II) by hand-sewing without using a Braun procedure.
In terms of TLDG, most of the procedures except for tumor location determination, specimen removal, and anastomotic reconstruction were similar to those of LADG. We always performed intraoperative gastrofiberoscopy prior to the gastric resection to localize the cancer lesion and acquire a sufficient tumor-free resection margin. The resected specimens were delivered through the umbilical port by transumbilical extension of the incision (from 2 to 3 cm). This extended transumbilical wound was protected by a double-ring wound protector (Alexis®; Applied Medical Resources Co., Rancho Santa Margarita, CA, USA). Most of the anastomotic reconstructions used the intracorporeal Billroth II (97.3%). Following the remake pneumoperitoneum, entry holes were made at the jejunum approximately 30 cm distal of Treitz's ligament and the greater curvature side of the stomach using a dissector with electrocautery. After the arms of the endoscopic linear stapler were inserted into the posterior wall of the remnant stomach and the antimesenteric side of the jejunum, the stapler was fired. The entry holes of the stomach and jejunum were closed with the stapler or a laparoscopic hand-sewn suture. When performing a gastroduodenostomy (Billroth I), we performed a side-to-side anastomosis using a linear stapler (the so-called "delta-shaped anastomosis") that was introduced by Kanaya et al.17 in 2002.
A χ2 test and a Student's t-test were conducted to compare nominal and continuous variables between groups, respectively. One-way analysis of variance was used for the moving average methods. The IBM® SPSS® Statistics version 20 software (IBM Corporation, Somers, NY, USA) was used to perform the analyses. Values of P<0.05 were considered significant.
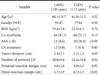
The demographics and clinicopathological information of the studied patients are summarized in Table 1. The groups were similar with respect to age (LADG vs. TLDG, 60.1 vs. 61.0), gender (1:0.49 vs. 1:0.57, respectively), body mass index (23.4 vs. 23.5, respectively), and co-morbidities (47.1% vs. 57.7%, respectively) (P>0.05). However, high ASA scores (>II) assessing the risk of surgery occurred more often in the TLDG group (8.6% vs. 26.8%, P<0.01).
No differences in pathological data were observed between groups for lymph node metastasis (LADG vs. TLDG, 8.8% vs. 6.3%), tumor size (2.5 cm vs. 2.2 cm, respectively), and proximal resection margin (4.8 cm vs. 4.9 cm, respectively; P>0.05). Significantly more lymph nodes were retrieved in the TLDG group (20.8 vs. 23.4; P=0.047). In addition, the distal resection margin lengths were significantly longer in the TLDG group (5.1 cm vs. 6.1 cm; P<0.01).
The mean operation time was significantly shorter in the TLDG group (LADG vs. TLDG, 300.2 min vs. 251.4 min; P<0.01). There were no deaths in either group. There were no significant differences in the open conversion rate (2.9% vs. 1.8%, respectively), combined resection (8.8% vs. 12.6%, respectively), reoperation rate (2.2% vs. 0.9%, respectively), or postoperative complications (14.7% vs. 18.8%, respectively; P>0.05). The mean time to first flatus (LADG vs. TLDG, 3.3 days vs. 3.1 days) and the mean hospital stay (15.5 days vs. 14.3 days, respectively) were similar between the 2 groups (P>0.05). In the surgical procedure, the extent of lymph node dissection was wider in the TLDG group than in the LADG group (D1+:D2, LADG vs. TLDG, 129:7 vs. 64:43; P<0.01). Furthermore, there were significant differences in gastrointestinal reconstruction type (BI:BII, 104:32 vs. 3:108; P<0.01; Table 2). In terms of postoperative complications, there were no remarkable differences between groups (P>0.05; Table 3).
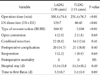
Table 4 compares values for gastric cancer before (60 patients) and after (120 patients) TLDG learning curve completion. The overall postoperative complication rate was significantly decreased after learning curve completion (43.3% vs. 19.0%; P<0.01). In particular, many intra-abdominal abscesses occurred before learning curve completion (13.3% vs. 1.7%; P<0.01). Comparison of other complications (e.g., leakage, bleeding, ileus, lung complications) showed a non-significant trend toward lower rates after learning curve completion than that before completion (P>0.05). There was a significant difference in moderate to severe postoperative complications (ASCPC≥2; 26.7% vs. 9.9%; P<0.01). The mean operation time was significantly shorter in the after completion group (before vs. after, 285.9 min vs. 254.4 min; P=0.01). Other clinical data (e.g., open conversion rate, reoperation rate, mortality, hospital stay, time to first flatus) were similar between the 2 groups (P>0.05).
Fig. 1 shows the mean values of operation time, hospital stay, and time to first flatus in each group. Viewing these 3 variables together, we identified the plateau or positive outcomes after 40 cases by surgeon A and 30 cases by surgeon B. These results indicated that experience with 40 and 30 TLDG cases was required to achieve optimum proficiency by the 2 surgeons, respectively.
The overall CUSUM failure rates were 13.2% (surgeon A, 16/121) and 8.47% (surgeon B, 5/59). Three phases could be defined in surgeon A's CUSUM curve. In the first phase, a positively sloping curve appeared until 30 cases, suggesting the process of overcoming the learning period. In the second and third phases, a plateau and a negatively sloping curve were seen after 30 cases, indicating learning curve completion. A slowing change was identified in surgeon B's CUSUM curve. However, we discovered that the CUSUM curve peaked around 20 cases (Fig. 2). These curves indicated that performing 30 and 20 TLDG cases was required to create the learning curve with these 2 surgeons. The moving average method and CUSUM curve results implicated that the learning curves were complete after 40 and 20 cases by these 2 surgeons.
Laparoscopic gastrectomy has become a standard surgical treatment for patients diagnosed with early gastric cancer in East Asia.1,2 Minimal invasiveness and post-surgical quality of life in patients with early gastric cancer have recently received much focus. As such, TLDG using intracorporeal anastomosis without a small skin incision is gaining popularity. This study analyzed the learning curve of TLDG and compared early surgical outcomes of TLDG and LADG after learning curve completion. We also conducted the comparison between before and after TLDG learning curve.
This analysis shows that the TLDG learning curves by the 2 surgeons were considered complete after 40 and 20 operations. We ascertained that 40 and 30 cases of operations by surgeons A and B, respectively, were the points of learning completion in the moving average method, whereas 30 and 20 cases by the surgeon A and B, respectively, were the points of learning completion in the CUSUM curves. The TLDG failure rate was higher for surgeon A (surgeon A vs. surgeon B, 13.2% vs. 8.5%). Hence, we selected 40 cases as surgeon A's learning period among the 40 and 30 cases from the 2 methods and 20 cases as surgeon B's learning period among the 30 and 20 cases from the 2 methods. In fact, surgeon B participated as a first assistant during the initial 20 TLDG cases performed by surgeon A. As such, surgeon B had the opportunity to learn practical technical tips for intracorporeal anastomosis, including appropriate retraction skills, the direction of the linear stapler, and how to handle the instruments best. These experiences of surgeon B quite likely resulted in the reduced learning period. Possible strategies to reduce the TLDG learning curve include assistance from other well-trained staff, close intraoperative supervision by an expert, completion of an animal workshop, and completion of simulator exercises for intracorporeal anastomosis.
Several LADG learning curve studies have been reported to date. These reports showed that experience in managing 40~60 cases of LADG with systemic lymphadenectomy for early gastric cancer was required to achieve proficiency and reach a learning curve plateau.6-9 In contrast, no studies have yet reported on TLDG learning curves. To our knowledge, this study is the first report to analyze the TLDG learning curve. We also analyzed the multidimensional learning curve using the moving average method and CUSUM. Multidimensional analysis of the learning curve is particularly useful, as its use allows several essential parameters to be put together in a single graph.4,10
Intraoperative identification of tumor location is a prerequisite for TLDG because of the need for appropriate planning of the extent of gastric resection. During LADG, surgeons can easily identify tumor locations and tumor-free margins under direct vision. However, it is impossible to localize a tumor directly in TLDG. We solved this problem by using intraoperative endoscopy. During the laparoscopic surgery, we performed simultaneous gastroendoscopy and could then mark the stomach wall with a felt-tip pen under both laparoscopic and endoscopic vision. Besides this, several methods have been reported to localize tumors during laparoscopic gastrectomy, such as intraoperative laparoscopic ultrasonography after preoperative endoscopic clipping,18 intraoperative portable plain radiography with endoscopic clipping,19 and endoscopic tattooing. 20,21
Another important finding of this study is that TLDG is a timesaving procedure. The mean operation time was significantly shorter in the TLDG group (300.2 min vs. 251.4 min; P<0.01). In our earlier study, we recommended the use of total laparoscopic procedures in patients with high body mass index values or thick abdominal walls.22 We believe that the mini-laparotomy skin incision and the process of securing a proper visual field during extracorporeal anastomosis are the major causes of this. However, these issues were not observed when we performed the intra-corporeal anastomosis. Lee et al.23 also reported that the intracorporeal Billroth II anastomosis time of TLDG was statistically shorter than that of LADG.
Overall and moderate to severe (ASCPC≥2) morbidity rates reduced significantly after learning curve completion. Morbidity rates (leakage, intra-abdominal abscess, bleeding, wound, and lung complications) decreased after learning curve completion as well. In particular, the decrease in intra-abdominal abscess rate was statistically significant (P<0.01). We defined intra-abdominal abscess as the presence of septic fluid in the abdominal cavity that resulted in pyrexia (body temperature >38℃) and was confirmed by ultrasonography or CT.24 For beginners and inexperienced TLDG surgeons, it is difficult to manage technical problems related to intra-corporeal anastomosis properly and to prevent contamination by the bowel contents while making the entry hole with the stapler. These initial experiences could cause early morbidities such as leakage, bleeding, and intra-abdominal abscesses. Inexperienced surgeons should pay particular attention to using proper anastomotic reconstruction techniques to prevent the occurrence of intra-abdominal abscesses until they overcome this learning curve.
Our study has several limitations. First, it was retrospective and nonrandomized. The enrolled patients underwent a number of different surgical procedures (Billroth I vs. Billroth II and hand sewing vs. linear stapler anastomosis). These factors would reflect a selection bias. Furthermore, detailed analysis about the timesaving effect in TLDG was lacking. Despite these drawbacks, we can ascertain some advantages. To our knowledge, the present study is the first report on the TLDG learning curve. It provides practical information for inexperienced TLDG surgeons by comparing learning curve completion data. We analyzed LADG and TLDG learning curve completion data to elucidate the feasibility of this newly extending surgical procedure further.
In conclusion, the learning curve was considered complete after 40~60 cases of TLDG in the training phase. The use of TLDG for early gastric cancer after learning curve completion was feasible and timesaving compared with LADG.
Figures and Tables
 | Fig. 1Mean operation time, hospital stay, and time to first flatus values according to the surgeon's level of TLDG experience. OP = operation; TLDG = totally laparoscopic distal gastrectomy. |
 | Fig. 2CUSUM analysis for TLDG in two surgeons. CUSUM = cumulative sum; TLDG = totally laparoscopic distal gastrectomy. |
Acknowledgments
The authors would like to thank Sung-Ho Jin at Korean Cancer Center Hospital for consultant about CUSUM method. We also thank Young-Suk Kim, RN for conducting data collection.
References
1. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010. 251:417–420.

2. Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007. 245:68–72.

3. Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008. 12:1015–1021.

4. Hosogi H, Kanaya S. Intracorporeal anastomosis in laparoscopic gastric cancer surgery. J Gastric Cancer. 2012. 12:133–139.

5. Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, et al. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011. 25:1076–1082.

6. Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007. 21:28–33.

7. Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005. 11:7508–7511.

8. Fujiwara M, Kodera Y, Miura S, Kanyama Y, Yokoyama H, Ohashi N, et al. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection: a phase II study following the learning curve. J Surg Oncol. 2005. 91:26–32.

9. Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc. 2009. 23:1259–1264.

10. Novick RJ, Stitt LW. The learning curve of an academic cardiac surgeon: use of the CUSUM method. J Card Surg. 1999. 14:312–320.

11. Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009. 250:177–186.

12. Williams SM, Parry BR, Schlup MM. Quality control: an application of the cusum. BMJ. 1992. 304:1359–1361.

13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010. 17:1471–1474.

14. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011. 14:101–112.
15. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011. 14:113–123.
16. Joo YT, Moon HG, Lee SH, Jeong CY, Jung EJ, Hong SC, et al. Laparoscopy-assisted distal gastrectomy with intracorporeal Billroth I stapled anastomosis using a hand access device for patients with gastric cancer. Surg Endosc. 2007. 21:859–862.

17. Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002. 195:284–287.

18. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc. 2005. 19:1353–1357.

19. Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, et al. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc. 2011. 25:958–963.

20. Tanimura S, Higashino M, Fukunaga Y, Osugi H. Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc. 2003. 17:758–762.

21. Jeong O, Cho SB, Joo YE, Ryu SY, Park YK. Novel technique for intraoperative tumor localization during totally laparoscopic distal gastrectomy: endoscopic autologous blood tattooing. Surg Endosc. 2012. 26:1778–1783.

22. Jeong SH, Lee YJ, Bae K, Ha WS, Park ST, Choi SK, et al. Clinical factors affecting the length of minilaparotomy incision in laparoscopy-assisted distal gastrectomy. J Laparoendosc Adv Surg Tech A. 2009. 19:129–133.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download