Abstract
As the incidence of early gastric cancer increases, laparoscopic surgery has become one of the treatments of choice for gastric cancer. With the increase of laparoscopic surgery, the chance of discovering aberrant anatomy during the operation also increases. We present a case of laparoscopic total gastrectomy in gastric cancer patients with intestinal malrotation. Intestinal malrotation occurs in one in every 500 births. We found that laparoscopic total gastrectomy in such patients can be performed successfully when it is performed with a proper Roux limb orientation through an alternative minilaparotomy.
Intestinal malrotation is a rare congenital anomaly referring to incomplete rotation of the primitive midgut around the superior mesenteric artery (SMA) during fetal development. Most intestinal malrotation patients present with symptoms of bowel obstruction during the first few months of life.1 However, some patients can be asymptomatic and are discovered incidentally during a radiographic study or surgical procedure.
When surgeons encounter intestinal malrotation unexpectedly, it can cause significant difficulties to performing standard procedures. The ability to perform a planned surgery in patients with aberrant anatomy relies on being aware of the anatomical variance and having the capability to modify the operation accordingly. Here, we present a case of a gastric cancer patient with intestinal malrotation who underwent laparoscopy-assisted total gastrectomy.
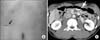
A 44-year-old male was diagnosed with early gastric cancer on a routine health evaluation. Physical examination was unremarkable, and laboratory findings showed no abnormalities. He had no abdominal pain, nausea, or vomiting. He did not receive any previous abdominal surgery. Esophagogastroduodenoscopy revealed gastric cancer at the upper body of the stomach (Fig. 1A). Contrast-enhanced computed tomography showed no demonstrable mass in the stomach. Additionally, incidental intestinal malrotation was revealed (Fig. 1B). He was scheduled for laparoscopic surgery after obtaining informed consent for the procedure.
After general endotracheal anesthesia was induced, an infraumbilical incision was made, and a 10-mm trocar was introduced into the peritoneal cavity using an open technique. Pneumoperitoneum was established, and other trocars were inserted under direct vision in the superior parts of the left upper quadrant (left upper port, 5 mm), right upper quadrant (right upper port, 5 mm), left flank (left lower port, 12 mm), and right flank (right lower port, 12 mm) of the abdomen. The locations of the trocars were the same as in other total gastrectomies performed at our institution. The operation was performed as previously described, with slight modification.2,3 The greater omentum was divided and dissected toward the lower pole of the spleen. The left gastroepiploic vessels and short gastric arteries were isolated and ligated. After dissection of the head of the pancreas, the right gastroepiploic vessels were ligated and divided. After ligation of right gastric artery, the duodenum was transected with a linear stapler. The left gastric vessels were exposed and divided for adequate lymph node dissection. Lymph nodes along the splenic vessels were cleared. After two laparoscopic bulldog clamps were applied on the distal esophagus, the esophagus was transected with an energy device. We used nonabsorbable 2-0 thickness monofilament with grasper and needle driver for purse string suture. After the purse-string suture at the esophagus was completed, the jejunum was identified under direct vision for the esophagojejunostomy. The small bowel was located on the right side of the abdomen, and the ligament of Treitz was absent (Fig. 2A). The appendix and colon were found on the left side of the patient (Fig. 2B). There were no Ladd's band and no vascular anomaly while preparing jejunal limb. Due to the altered small bowel configuration, we modified the minilaparotomy site. We extended the right lower port site up to 3.5 cm rather than in the usual left lower port site to account for the intestinal malrotation. The previously identified jejunum was brought out and transected with a linear stapler. A jejunojejunostomy was made 50 cm distal to the tentative esophagojejunostomy site. A circular stapler was inserted into the transected jejunum. After extracorporeal installation of anvil to the circular stapler, the prepared jejunum with circular stapler were introduced into the peritoneal cavity (Fig. 2C). When the anvil was inserted in the esophagus appropriately, a previously made pursestring suture was tied, and a circular stapler was fired. The jejunal stump was closed with a linear stapler. The jejunojejunostomy remained in the right upper quadrant. The Roux limb was brought up in a 'reverse C fashion' due to the abnormal anatomy (Fig. 2D). We did not perform appendectomy. The patient recovered without any complications. He was discharged on postoperative day 5. At the six-month outpatient follow-up, he was recovering well and tolerating a regular diet (Fig. 3).
Intestinal malrotation is an embryologic anomaly resulting from incomplete rotation of the embryologic gut around the axis of the SMA. The incidence of malrotation in adults is rare, estimated to be approximately 0.2% of the population. Most cases are diagnosed in infants and children.1
To understand intestinal malrotation, knowledge of intestinal embryology is essential. The primitive gut in the early embryo is a straight tube that consists of the foregut, midgut, and hindgut. The midgut starts to elongate and rotate. This process has been divided into three steps. First, the midgut herniates into the celom of the body stalk at the sixth week of gestation, undergoing a counterclockwise rotation of 90° so that the duodenojejunal loop lies on the right, and the cecocolic loop lies to the left of the SMA axis. The second step occurs in gestation week 10 and involves further counterclockwise rotation of the midgut within the abdominal cavity, completing a 270° rotation. This rotation brings the duodenal 'c' loop behind the SMA with the ascending colon to the right, the transverse colon above, and the descending colon to the left. The third step involves fusion and anchoring of the mesentery. The cecum descends, and the ascending and descending colon attach to the posterior abdomen.4
Intestinal malrotation results when midgut rotation is arrested prematurely. Three types of malrotation have been described. Type I malrotation occurs when normal midgut rotation ceases at 6 weeks, after 90° of rotation; the proximal small bowel is on the right, and the cecum is on the left. In type II malrotation, the derangement in rotation occurs between 6 and 10 weeks and disrupts duodenal rotation. An error after 10 weeks results in type III malrotation, in which the duodenum only completes 90° of additional rotation. Fibrous bands called Ladd's bands crossing over the second portion of the duodenum connect the cecum to the right upper quadrant.5
When intestinal malrotation is symptomatic, elective operative intervention is indicated. The Ladd's procedure is the standard of care for resolving symptoms and preventing future complications. The Ladd's procedure includes untwisting the volvulus counterclockwise, division of the abnormal coloduodenal Ladd's bands, widening of the mesenteric base to prevent further volvulus, and appendectomy.6 An appendectomy is performed because of the atypical localization of pain and tenderness of acute appendicitis in the setting of intestinal malrotation. However, the management of asymptomatic intestinal malrotation patients diagnosed incidentally remains unclear.4,7,8
In the present case, intestinal malrotation was diagnosed incidentally by preoperative abdomino-pelvic computed tomography. Before the diagnosis, the patient had no history of abdominal complaints. Laparoscopic surgery for gastric cancer was accepted because of short-term advantages, such as reduced pain, shorter hospital stays, and early recovery, over conventional open surgery.9-11 The laparoscopic Ladd's procedure for the treatment of patients who have intestinal malrotation without midgut volvulus is also safe and effective.12,13 Thus, we scheduled the patient for laparoscopic surgery.
A few alterations were made in the standard approach to the total gastrectomy with Roux-en-Y esophagojejunostomy because of intestinal malrotation. Standard Ladd's procedure was not necessary in this case. There were no Ladd's bands, and the small bowel was already found on the right side and the colon on the left side. We modified the minilaparotomy site and Roux limb rotation. To avoid mesenteric twisting, we performed esophagojejunostomy with the Roux limb in a clockwise rotation instead of the routine counterclockwise rotation.
In laparoscopic surgery, the adequate angle to stapler access is core to perform successful intracorporeal bowel anastomosis. Thus, many laparoscopic procedures have standard trocar and stapler insertion angle. However, when performing gastrectomy to malrotated patient, the standard minilaparotomy for esophagojejunostomy may lead to mesenteric twisting or tension to anastomosis site. To perform adequate anastomosis to the patient of presented case, we made minilaparotomy site to opposite to the usual insertion site to overcome such difficulties. To our knowledge, this is the first reported case of laparoscopic total gastrectomy for gastric cancer patients with intestinal malrotation.
In conclusion, in the setting of the incidental finding of asymptomatic intestinal malrotation during gastric cancer surgery, a laparoscopic approach can be conducted successfully when it is performed with a proper Roux limb orientation through an alternative minilaparotomy.
Figures and Tables
Fig. 1
Preoperative evaluation. (A) Esophagogastroduodenoscopy. (B) Computed tomography axial view. Small bowel is located on the right side of the abdomen. The superior mesenteric vein (long white arrow) is located on the left side of the superior mesenteric artery (short white arrow). (C) Coronal view. Ileocecal valve (arrow) is on the left side of the abdomen.

References
1. Kapfer SA, Rappold JF. Intestinal malrotation-not just the pediatric surgeon's problem. J Am Coll Surg. 2004; 199:628–635.

2. Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008; 207:e6–e11.
3. Kim HI, Cho I, Jang DS, Hyung WJ. Intracorporeal esophagojejunostomy using a circular stapler with a new purse-string suture technique during laparoscopic total gastrectomy. J Am Coll Surg. 2013; 216:e11–e16.

4. Gohl ML, DeMeester TR. Midgut nonrotation in adults. An aggressive approach. Am J Surg. 1975; 129:319–323.
5. Stewart DR, Colodny AL, Daggett WC. Malrotation of the bowel in infants and children: a 15 year review. Surgery. 1976; 79:716–720.
6. Swenson O, Ladd WE. Surgical emergencies of the alimentary tract of the newborn. N Engl J Med. 1945; 233:660–663.

7. Gilbert HW, Armstrong CP, Thompson MH. The presentation of malrotation of the intestine in adults. Ann R Coll Surg Engl. 1990; 72:239–242.
8. Spigland N, Brandt ML, Yazbeck S. Malrotation presenting beyond the neonatal period. J Pediatr Surg. 1990; 25:1139–1142.

9. Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009; 146:469–474.

10. Park DJ, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012; 26:1548–1553.

11. Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopicassisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013; 27:2598–2605.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download