Abstract
Neurofibromas are benign tumors that originate from the peripheral nerves, including neurites and fibroblasts. Generally, a solitary neurofibroma is located in the skin and rarely in other places. A 72-year-old female suffered from epigastric discomfort for 2 months. Endoscopic findings showed an early gastric cancer type IIc at the antrum. Abdominal computed tomography revealed early gastric cancer with a 1.6 cm-sized metastatic node posterior to the duodenum. Laparoscopic assisted distal gastrectomy and retro-pancreatic dissection were performed uneventfully. Histological examination revealed gastric adenocarcinoma, invading the mucosa without nodal metastasis, and a neurofibroma. Herein, we present a case of a gastric cancer patient with a solitary retroperitoneal neurofibroma which mimicked a distant metastatic node.
Compared to advanced gastric cancer, early gastric cancer (EGC) shows a favorable prognosis, with 5-year survival rates exceeding 94%.1
Lymph node (LN) status is the most important prognostic factor. Even in EGC, the incidence of LN metastasis exceeds 10%; is reported to be 14.1% overall and appears in 4.8 to 23.6% of cases depending on cancer depth.2 It is important to evaluate LN status preoperatively for proper treatment strategy; however, sufficient results are not being obtained using various evaluation modalities.
According to Japanese staging guideline, N3 level node was regarded as distant metastasis.3 It is possible to cure local disease without distant metastasis by gastrectomy and LN dissection. However, there is no survival benefit from surgery for systemic disease with distant metastasis such as para-aortic LN metastasis.4 Therefore, whether the disease is local or systemic is an important prognostic indicator for gastric cancer, and the debate continues over the importance of extended lymphadenectomy for gastric cancer.
Herein, authors present a case misdiagnosed as inoperable EGC.
A 72-year-old woman, with no history of neurofibromatosis or other systemic disease, was referred to Chosun University Hospital (Gwangju, Korea) for EGC. She had undergone laparoscopic cholecystectomy several years previously.
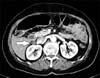
Abdominal computed tomography did not show the gastric tumor lesion but revealed a well-defined, 1.6 cm sized ovoid retroperitoneal mass located posterior to the duodenum (Fig. 1). Before her operation, she was diagnosed as EGC with distant nodal metastasis posterior to the duodenum.
The patient was placed in the supine position under general anesthesia. An operator and scopist stood to the right side of the patient, and an assistant surgeon stood to the left of the patient. A total of 5 trocars (two 12-mm trocars and three 5-mm trocars) were used. The first dissection began with the mobilization of the duodenum from hepatoduodenal ligament and right gastrocolic ligament. An assistant retracted the duodenum left laterally, and an operator dissected between the pancreaticoduodenal unit and inferior vena cava (IVC) and right renal vein. The mass was easily exposed by a sharp dissection and removed (Fig. 2). Several vessels from the mass to the IVC were ligated by the LigaSure™ vessel sealing system (Valleylab, Boulder, CO, USA).
Frozen biopsy reported that there was no adenocarcinoma component. After this report, laparoscopic distal gastrectomy with gastrojejunstomy was performed. Operative time was 3 hours and 40 minutes.
The final pathological diagnosis was a gastric adenocarcinoma that invaded a mucosa (T1a) without nodal metastasis (0/20, N0) and neurofibroma with immunohistochemical stains positive for S-100 protein (Fig. 3).
About 15% of EGC have LN metastases.2 This makes the prognosis of EGC better than advanced gastric cancer. Nevertheless, while there is little chance of nodal or distant metastasis in EGC, some case reports revealed stage IV EGC accompanied by extensive LN and distant metastasis.5-8
Accurate assessment of LN status is of crucial importance for appropriate treatment planning and determining prognosis in gastric cancer. But abdominal ultrasonography (AUS), endoscopic ultrasonography, multidetector-row computed tomography (MDCT), magnetic resonance imaging (MRI), and 18F-fluoro-2-deoxyglucose positron emission tomography cannot reliably be used to confirm or exclude the presence of LN metastasis.9
In this case, preoperatively the patient was diagnosed as EGC with distant metastasis by MDCT. But a pathological report indicated mucosa-confined adenocarcinoma (T1a) without nodal metastasis (N0) and solitary neurofibroma.
A neurofibroma is a neurogenic tumor, which arises from the nerve sheath. Typically, neurofibromas grow slowly and have minimal potential for malignant transformation unless they are associated with neurofibromatosis type 1 (formerly known as von Recklinghausen disease). A solitary neurofibroma is rare in cases without neurofibromatosis. Specifically, solitary retroperitoneal neurofibromas were studied in several case reports.10-12 Neurofibroma is more common in men, particularly in the 20~40 year age group.13 But in this case, patient was a 72-year old aged woman.
Retroperitoneal masses not arising from major solid organs are uncommon. Because the treatment options vary, it is useful to be able to differentiate these masses by using imaging criteria.14 Currently, imaging modalities such as MDCT, AUS and MRI show progress technically. But there is still a considerable overlap of imaging findings for these masses, and histological examination is often required for definitive diagnosis.14 Additionally, in gastrointestinal tract malignancy, a retroperitoneal mass is easily confused with LN metastasis.
In conclusion, staging laparoscopy with frozen biopsy might be helpful in treating a preoperatively EGC with distant metastasis.
Figures and Tables
Fig. 1
Abdomen computed tomography. Computed tomography shows a 1.6 cm-sized metastatic node (arrow) posterior to duodenum.
References
1. Hyung WJ, Kim SS, Choi WH, Cheong JH, Choi SH, Kim CB, et al. Changes in treatment outcomes of gastric cancer surgery over 45 years at a single institution. Yonsei Med J. 2008; 49:409–415.

2. Roviello F, Rossi S, Marrelli D, Pedrazzani C, Corso G, Vindigni C, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol. 2006; 94:275–280.

3. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition. Gastric Cancer. 1998; 1:10–24.
4. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008; 359:453–462.

5. Choi JY, Kim JI, Choi YC, Jun SY. Two cases of histopathologically advanced (stage IV) early gastric cancer. Korean J Gastroenterol. 2005; 45:64–67.
6. Shiomi M, Kamisako T, Yutani I, Kudo M, Shigeoka H, Tanaka A, et al. Two cases of histopathologically advanced (stage IV) early gastric cancers. Tumori. 2001; 87:191–195.

7. An JY, Choi MG, Noh JH, Kim KM, Kim DS, Sohn TS, et al. Stage IV early gastric cancer: two cases with microsatellite instability. Langenbecks Arch Surg. 2008; 393:105–109.

8. Kakushima N, Kamoshida T, Hirai S, Hotta S, Hirayama T, Yamada J, et al. Early gastric cancer with Krukenberg tumor and review of cases of intramucosal gastric cancers with Krukenberg tumor. J Gastroenterol. 2003; 38:1176–1180.

9. Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009; 12:6–22.

10. Poon JC, Ogilvie T, Dixon E. Neurofibroma of the porta hepatis. J Hepatobiliary Pancreat Surg. 2008; 15:327–329.

12. Plaza JA, Wakely PE Jr, Suster S. Lipoblastic nerve sheath tumors: report of a distinctive variant of neural soft tissue neoplasm with adipocytic differentiation. Am J Surg Pathol. 2006; 30:337–344.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download