Abstract
Gastric cancer patients with acute disseminated intravascular coagulation experiences a rare but severe complication resulting in a dismal prognosis. We report a case of advanced gastric cancer complicated with disseminated intravascular coagulation with intractable tumor bleeding which was successfully treated with chemotherapy consisting of 5-fluorouracil and oxaliplatin. The patient was a 63-year-old man who complained of abdominal pain, melena, and dyspnea on 24 November 2010. We diagnosed stage IV gastric cancer complicated by disseminated intravascular coagulation. Gastric tumor bleeding was not controlled after procedures were repeated three times using gastrofiberscopy. With the patient's consent, we selected the 5-fluorouracil and oxaliplatin combination chemotherapy for treatment. After one cycle of 5-fluorouracil and oxaliplatin therapy, symptoms of bleeding improved and the disseminated intravascular coagulation process was successfully controlled. The primary tumor and multiple metastatic bone lesions were remarkably shrunken and metabolically remitted after eight cycles of chemotherapy. In spite of progression, systemic chemotherapy is effective in disease control; further, the patient gained the longest survival time among cases of gastric cancer with disseminated intravascular coagulation.
Gastric cancer is the fifth most common malignant tumor and ranks second for cancer deaths worldwide.1 Disseminated intravascular coagulation (DIC) is a pathologic syndrome in which the manifestations, such as progressive and consumptive coagulopathy with severe bleeding, are in large part a consequence of thrombin formation.2 Solid tumors such as gastrointestinal, pancreatic, liver, ovarian, breast, lung and prostatic carcinomas can induce acute DIC complication.3 The gastric cancer patient with acute DIC experiences a rare but severe complication resulting in a very dismal prognosis. DIC associated with gastric adenocarcinoma occurs through the exacerbation of the blood clotting process by which procoagulant materials such as mucin extracts derived from tumor cells directly stimulate coagulation factor X or damage red blood cells and platelets by direct contact with tumor cells on microvessel. Active tumor treatment improves DIC, rather than palliative treatments such as coagulation factor and platelet transfusions. Choosing the appropriate chemotherapy agents to treat the underlying cancer and stop the acute DIC process effectively, while avoiding chemotherapy induced myelosuppresion which may contribute to bleeding related mortality, is difficult. However, many clinicians tend to be reluctant to apply palliative chemotherapy due to potent adverse events combined with intrinsic hematologic fragility. Because we experienced a case of proven metabolic complete remission in which chemotherapy significantly improved the gastric cancer and thereby the accompanying DIC, we report this case.
The 63-year-old man was admitted to the hospital on 24 November 2010 with epigastric pain, diffuse back pain and melena. The physical examination showed poor general condition (Eastern Cooperative Oncology Group performance grade 3) and petechia in lower extremities. He had blood pressure of 140/80 mmHg, pulse rate of 100 beats/min, respiratory rate of 22 beats/min, body temperature of 36.6℃ and oxygen saturation of 95% in room air upon admission. There was no anemia of conjunctiva or jaundice of sclera and both neck and chest were normal. The blood count showed hemoglobin of 12.1 g/dl, leukocytes of 6,900/mm3 and platelet count of 24,000/mm3. Liver function tests showed total protein of 6.6 g/dl, albumin of 4.0 g/dl, total bilirubin of 1.6 mg/dl, aspartate aminotransferase (AST) of 268 IU/L, alanine aminotransferase (ALT) of 36 IU/L and alkaline phosphatase of 214 IU/L. Blood coagulation tests revealed prolonged prothrombin time (PT 1.53 international normalized ratio, 56%), elevated fibrinogen degradation products level (FDP 66.5 µg/ml), D-dimer (21.27 µg/ml) and lactate dehydrogenase level (LDH 2,116 IU/L). HBsAg and hepatitis C virus antibody showed negative findings. Upper gastrointestinal endoscopy performed in our hospital found a Bormann type 2 mass in the lower body. Because there was bleeding on a vessel exposed to the area of mass, argon plasma coagulation was performed (Fig. 1A). Endoscopic biopsy results were poorly differentiated adenocarcinoma. Abdominal computed tomography scanning revealed gastric cancer in the lower body and multiple metastases in neighboring lymph nodes, and abdominal lymph nodes were observed. Liver and bilateral adrenal metastases were observed but there was no evidence of cirrhotic change. We could exclude other primary liver diseases. Positron emission tomography scanning found an increase in uptake of fluorodeoxyglucose in gastric area. Liver, bilateral adrenal and multiple bone metastases were observed as well (Fig. 2A). We diagnosed stage IV gastric cancer having multiple bone metastases complicated by DIC. Gastric tumor bleeding was not controlled after three repeated procedures using gastrofiberscopic intervention with argon plasma coagulation. Repeated packed red blood cell (RBC), fresh frozen plasma and platelet concentrate transfusions, DIC with pancytopenia resulted in more aggravated. With consent of the patient and his family, we performed 5-fluorouracil (5-FU) and oxaliplatin combination chemotherapy on 1 December 2010 to control the underlying gastric cancer. Systemic combination chemotherapy was composed of 5-FU 750 mg/m2 on day 1, 2 and day 22, 23, oxaliplatin 75 mg/m2 and leucovorin 75 mg/m2 on day 1 and day 22. Chemotherapy was repeated every four weeks. After one cycle of the 5-FU and oxaliplatin therapy, the bleeding symptoms improved, and the DIC process was successfully controlled. At that time, the hematologic profile improved but did not resolve to normal: white blood cell 4,800/mm3, hemoglobin 12.0 g/dl, platelet 117,000/mm3. Liver function tests showed total protein of 7.7 g/dl, albumin of 4.1 g/dl, total bilirubin of 0.8 mg/dl, AST of 35 IU/L, ALT of 32 IU/L and alkaline phosphatase of 316 IU/L. He felt better than before chemotherapy, melena stopped and the petechiae of lower extremities disappeared. His back pain, previously uncontrolled by high dose opioid administration, dramatically improved after chemotherapy.
Chemotherapy was treated 6 times in a total of 6 months. Traced abdominal computed tomography scanning, positron emission tomography scanning and upper gastrointestinal endoscopy (Fig. 1B) showed complete remission of gastric cancer, lymph nodes and metastases areas (Fig. 2B).
After 8 cycles of initial combination therapy, although we recommended continuing chemotherapy until progression, he refused further chemotherapy. Thereafter, we observed him without systemic chemotherapy at regular follow up 3 week intervals. During the observation period he did not showed abnormal hematologic profile and back pain and there was no evidence of progression in abdominal computed tomography scan and follow up gastrofiberscopy. After 4 months of observation, he suffered severe back pain without hematologic abnormality. In the positron emission tomography-computed tomography (PET-CT) scan, aggravated multiple spine lesions were detected (Fig. 2C). We performed 5-FU, leucovorin and irinotecan combination chemotherapy on 3 December 2011 to control the metastatic lesion. Systemic combination chemotherapy was composed of 5-FU 1,000 mg/m2 on day 1, 2 and day 22, 23, leucovorin 200 mg/m2 on day 1 and day 22, irinotecan 180 mg/m2 on day 1 and day 22. Chemotherapy was repeated every four weeks. During the 2nd line chemotherapy, laboratory tests revealed no evidence of DIC. After 4 cycles of second line chemotherapy, follow up PET-CT scan showed multiple spine lesions improved (Fig. 2D). But after 6 cycles of second line chemotherapy, PET-CT scan showed that multiple bone metastases and both adrenal metastases were aggravated (Fig. 2E). We performed tegafur+gemeracil+oteracil potassium (TS-1), cisplatin combination chemotherapy on 8 August 2012 to control the metastatic lesion. Systemic combination chemotherapy was composed of TS-1 60 mg for two weeks, cisplatin 75 mg/m2 on day 1, every 3 weeks. The blood count showed hemoglobin of 10.9 g/dl, leukocytes of 3,100/mm3 and platelet count of 115,000/mm3. Liver function tests showed total protein of 6.1 g/dl, albumin of 3.3 g/dl, total bilirubin of 0.8 mg/dl, AST of 56 IU/L, ALT of 25 IU/L and alkaline phosphatase of 611 IU/L. He remains free of DIC up to the present time. In the course of chemotherapy he did not experience febrile neutropenia or overt systemic infection. He is now treated with 3rd cycle of TS-1 and cisplatin without complications.
Gastric cancer that initially presents with DIC is rare. A few studies reported that gastric cancer with an initial presentation of DIC had a dismal prognosis. Our case suggested that survival and control of hematologic complication of initially DIC combined gastric cancer might be improved by early and intensive systemic chemotherapy.
Acute DIC is a pathologic syndrome in which the manifestations, such as progressive and consumptive coagulopathy with severe bleeding, are in large part a consequence of thrombin formation.2 Solid tumors may induce acute DIC complication.3 Treatment consisting of fresh frozen plasma and platelet replacement, with or without a heparin injection, had little clinical effect. DIC is a classic but rare complication of solid tumors, and gastric cancer is one of the most frequent causes. The short-term prognosis remains very poor, but palliative chemotherapy may prolong survival.4 Most of gastric cancer with DIC studies included small numbers of patients (5 to 18) because of the rarity of the condition.5-7 Furthermore, there have been few studies comparing prognosis between patients who receive best supportive care and palliative chemotherapy. Survival is in the order of a few weeks, particularly in the event of bone marrow invasion, which is frequent in the histological subtype with signet ring carcinoma cells.4,8 In some retrospective studies of patients with gastric cancer with bone metastases, the survival of the patients presenting with DIC was markedly shorter than that of the patients free from hematological complications.4,9 The poor prognosis is related to impairment of the general condition, reflecting advanced metastatic disease, and the hemorrhagic complications of DIC. Myelosuppressive chemotherapy in the context of coagulopathy and pancytopenia due to bone marrow invasion is usually poorly tolerated and may worsen the initial prognosis through hemorrhagic or infectious complications.
In metastatic gastric cancer, combination chemotherapy has been primarily used10,11 and the most commonly used drugs are cisplatin and 5-FU.10 In this case, hematologic complications were not controlled by conservative treatment including repeated endoscopic bleeding control and RBC, platelet and frozen fresh plasma supplements due to uncontrolled underling provocation events of gastric cancer bleeding. The main key of treatment of DIC is the recovery of the provoking factor; so we should consider first the treatment of gastric cancer bleeding control. Systemic chemotherapy is the primary treatment mode for a large portion of recurrent or metastatic gastric cancer; the most commonly used drugs are cisplatin and 5-FU,10 but there are no clear reports although many studies have assessed which treatment agent is the best. Usually in our institution, the most commonly-used first-line chemotherapy regimen is fluoropyrimidine and platinum combination, which is followed by taxane and platinum combination. So the treatment regimen for the current patient was not different from that of metastatic gastric cancer without DIC. Fluoropyrimidine derivatives that could be used include 5-FU, capecitabine, and tegafor+gemeracil+oteracil potassium (TS-1). Platinum options are cisplatin, carboplatin and oxaliplatin. Oral chemotherapeutic agents such as TS-1 and capecitabine present difficulties in maintaining effective concentration in the blood. In the case reported herein, the 5-FU and oxaliplatin combination chemotherapy regimen was administered since the activity of this combination has been demonstrated in phase II and III clinical trials in metastatic gastric cancer,12,13 with a good safety profile even in patients with impaired general condition. The rapid response of clinical symptoms including bone and abdominal pain and laboratory (DIC) findings were authenticated by the complete lymph node response imaged by PET scan. The good safety profile of 5-FU and oxaliplatin enables optimal sequences to be used from the outset in advanced cases with concomitant diseases and impaired general condition, thus achieving a faster tumor response. In our knowledge, the case reported herein is the second case of metastatic gastric cancer with DIC to be treated using the 5-FU and oxaliplatin combination regimen.14 A good-quality tumor response was induced with rapid control of the DIC and a very satisfactory safety profile.
This case showed that combination administration of oxaliplatin, leucovorin and 5-FU improved DIC and produced clinically complete remission in case of DIC, liver metastasis, bone metastasis and aortic lymph node metastasis. To our knowledge, this patient is the longest survivor whose initial presentation was metastatic gastric cancer combined with DIC, with survival at more 22 months. Most reported cases ultimately died of disease progression and re-developed DIC.15 In this case, the event provoking DIC was tumor bleeding. We should consider more aggressive treatment for underlying disease to stop the vicious cycle of bleeding and consumptive DIC, because we can predict more favorable outcome DIC provoked with tumor bleeding. This is only one case report, but our report suggests that early and intensive management for DIC and systemic chemotherapy improves prognosis in this clinical setting. This case suggests that more effective and less toxic regimens are needed.
In general, performance status and organ functions are most important factor when consider palliative chemotherapy in metastatic cancer patients for safety and tolerability. This case was not satisfied the performance status but, showed that systemic chemotherapy could control the tumor bleeding control and the complication of DIC, and eventually prolongs survival beyond prediction. Therefore, early and intensive management for correctable DIC followed by systemic chemotherapy should be considered in patients with DIC like this patient. There are several equivalent of combination of first line chemotherapy in case of advanced gastric cancer, among them; 5-FU and oxaliplatin combination could be a safe and effective chemotherapy option in DIC complicated gastric cancer patients because of the rapid, effective tumor response which was induced with rapid control of the DIC. Therefore, early and intensive management for correctable DIC followed by chemotherapy should be considered for patients with gastric cancer who initially present with DIC for improving prognosis.
Figures and Tables
Fig. 1
Initial (A) and follow up (B) gastroscopic finding: (A) Ulcerofungating mass with spontaneous oozing on the posterior wall of mid body. (B) Ulcer scar without definite mass-like lesion, and biopsy finding revealed chronic active gastritis with mild intestinal metaplasia without tumor cells.
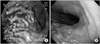
Fig. 2
(A) Initial positron emission tomography-computed tomography (PET-CT) scan showed stomach cancer in body, multiple perigastric, periaortic, multiple liver metastases, and disseminated bone metastasis. (B) After 6 cycles of first line chemotherapy, follow up PET-CT scan showed nearly complete remission of stomach cancer and metastatic sites. (C) After 4 months of drug vacation, PET-CT scan showed multiple spine lesions were aggravated. (D) After 4 cycles of second line chemotherapy, PET-CT scan showed multiple spine lesions improved. (E) After 6 cycles of second line chemotherapy, PET-CT scan showed that multiple bone metastases and both adrenal metastases were aggravated.
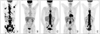
References
1. Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003. 362:305–315.
2. Colman RW, Rubin RN. Disseminated intravascular coagulation due to malignancy. Semin Oncol. 1990. 17:172–186.
3. Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, Cavazzini G, et al. Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology. 1995. 52:505–508.

4. Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010. 25:1537–1542.

5. Yeh KH, Cheng AL. Gastric cancer associated with acute disseminated intravascular coagulation: successful initial treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and leucovorin. Br J Haematol. 1998. 100:769–772.

6. Tokar M, Bobilev D, Ariad S, Geffen DB. Disseminated intravascular coagulation at presentation of advanced gastric cancer. Isr Med Assoc J. 2006. 8:853–855.
7. Hironaka SI, Boku N, Ohtsu A, Nagashima F, Sano Y, Muto M, et al. Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with bone metastasis. Gastric Cancer. 2000. 3:19–23.

8. Kusumoto H, Haraguchi M, Nozuka Y, Oda Y, Tsuneyoshi M, Iguchi H. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep. 2006. 16:735–740.

9. Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, Seo Y, et al. Diffuse bone metastasis with hematologic disorders from gastric cancer: clinicopathological features and prognosis. Oncol Rep. 1999. 6:601–605.

10. Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. A combination immunochemotherapy of 5-fluorouracil, cisplatin, leucovorin, and OK-432 for advanced and recurrent gastric carcinoma. Hepatogastroenterology. 2003. 50:2259–2263.
11. Makino H, Naito K, Tsuruta A, Kan K, Toda S, Yoshimura N, et al. A case of complete remission of metastatic skin carcinoma (erythema type) from advanced gastric cancer by CDDP administration. Gan To Kagaku Ryoho. 1993. 20:2225–2228.
12. De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005. 92:1644–1649.

13. Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008. 26:1435–1442.

14. Ferrand FR, Gontier E, Guymar S, Fagot T, Ceccaldi B, Malfuson JV, et al. Effectiveness and safe use of modified FOLFOX-6 for metastatic gastric cancer with signet ring cell components complicated by disseminated intravascular coagulation and diffuse bone marrow carcinomatosis. Onkologie. 2012. 35:118–120.

15. Huang TC, Yeh KH, Cheng AL, Hsu CH. Weekly 24-hour infusional 5-fluorouracil as initial treatment for advanced gastric cancer with acute disseminated intravascular coagulation. Anticancer Res. 2008. 28:1293–1297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download