Abstract
Purpose
Chronic inflammation induces cancer and cancer induces local tissue damage with systemic inflammation. Therefore, the aim of this study is to investigate the potential relationship between the severity of inflammation and prognosis in cancer patients.
Materials and Methods
This study enrolled 220 patients from January 2002 to December 2006 who underwent gastric surgery. We evaluated the relationship between preoperative inflammatory parameters (erythrocyte sedimentation rate, neutrophil-to-lymphocyte ratio) and other clinicopathological factors. Survival outcomes were compared according to the extent of inflammation.
Results
Significant elevation of erythrocyte sedimentation rate was related with old age, increased neutrophil-to-lymphocyte ratio, decreased hemoglobin, increased carcinoembryonic antigen, increased tumor size and advanced TNM stage. Neutrophil-to-lymphocyte ratio was significantly correlated with old age, increased erythrocyte sedimentation rate and advanced TNM stage. In the univariate analysis, elevated erythrocyte sedimentation rate and increased neutrophil-to-lymphocyte ratio had significantly poorer survival than those without elevation (all P<0.05). However, the multivariate analysis failed to prove erythrocyte sedimentation rate and neutrophil-to-lymphocyte ratio as independent prognostic factors.
Conclusions
The elevation of erythrocyte sedimentation rate and neutrophil-to-lymphocyte ratio were correlated with poor prognosis in the univariate analysis and there was a strong correlation between inflammatory parameters (erythrocyte sedimentation rate and neutrophil-to-lymphocyte ratio) and tumor progression. Thus, erythrocyte sedimentation rate and neutrophil-to-lymphocyte ratio are considered useful as follow-up factors.
Gastric cancer is one of the most common malignancies and the third leading cause of cancer-related deaths in men and the second leading cause of cancer-related deaths in women in Korea.1 Several studies have shown the close relationship between gastric cancer and chronic inflammation, and the pathogenesis of gastric cancer presents as an inflammation-driven malignancy.2 Persistent infectious agents in the host induce chronic inflammation, which result in repeated cycles of cell and DNA damage and promote the growth of malignant cells.3 Although the exact mechanism is not clear, several articles suggested that tumor could induce inflammation in the host.4 Growth and invasion of the cancer induces local tissue damage and causes systemic acute phase response.4 So based on the fact that there is a close relationship between inflammation and cancer progression,5-8 we hypothesized that the progression of inflammation reflects the progression of cancer and predicts the prognosis of patients with cancer.
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are widely used as biomarkers for inflammation. There are several studies on the relationship between CRP and gastric cancer,9,10 but very few studies on ESR and gastric cancer.11 Recently, with regards to the correlation between inflammation and prognosis of cancer patients, many studies on neutrophil-to-lymphocyte ratio (NLR) have been performed.6,7
So, in our series, we analyzed the correlation between inflammatory parameters (ESR and NLR) and other clinicopathologic factors (Gender, Age, hemoglobin [Hb], carcinoembryonic antigen [CEA], CA19-9, tumor size and TNM stage). Second, we studied the clinical significance of ESR and NLR as prognostic indicators in gastric cancer patients.
We retrospectively analyzed 258 consecutive patients from January 2002 to December 2006, who had been operated on for gastric cancer. Among them, 38 patients who had palliative operations or data loss were excluded. Therefore, 220 patients were enrolled in this study. The Institutional Review Board of Seoul Paik Hospital approved this study (IIT-2012-360).
Pathologic staging was based on the TNM staging system of the American Joint Committee on Cancer (AJCC), 2010.
The measurement of serum ESR, white blood cell (WBC) count and tumor markers (CEA and CA19-9) were performed preoperatively. ESR was assessed by the modified Westergren method, which was done by transferring 0.8 ml of potassium-ethylenediaminetetraacetic acid (EDTA) blood into a vial containing a defined amount of 3.8% sodium citrate (0.2 ml) solution. The upper limit of normal for ESR was 10 mm/h in men and 20 mm/h in women.
Absolute neutrophil count (ANC) was derived by multiplying the WBC count times the percentage of neutrophils in the differential WBC count. Absolute lymphocyte count (ALC) was calculated by multiplying the WBC count times the percentage of lymphocytes. The NLR was defined as the ANC divided by the ALC. The cut off value for NLR was 2.15 (the 75th percentile).
Serum concentrations of CEA and CA19-9 were measured using a radioimmunoassay analysis. The cut off values for CEA and CA19-9 were 5 ng/ml and 37 U/ml.
Chi-square test was performed to evaluate the relationship between ESR and NLR and other clinicopathological factors. Cumulative survival curves were created using the Kaplan and Meier method. The log-rank test was used to compare the differences between survival curves according to variables. We used a Cox proportional hazards model for the multivariate analysis to establish the independent prognosticators. Data were analyzed by using statistical software (SPSS 15.0; SPSS Inc., Chicago, IL, USA) and P<0.05 was considered statistically significant.
Patients ranged in age from 23 to 89 years (mean age: 57 years), with 149 (67.7%) men and 71 (32.3%) women. Total gastrectomy and subtotal gastrectomy were performed on 88 and 132 patients, respectively. One hundred and seven patients had early gastric cancer and 113 patients had advanced gastric cancer (Table 1).
ESR was checked in 220 patients. 75 patients had an elevated preoperative ESR, and 145 did not. The median value was 13.26 mm/h (range, 1~138 mm/h).
Fifty-six patients had an increased NLR (≥2.15), and 164 patients were in the normal range. The median NLR was 1.82 (range, 0.55~7.54).
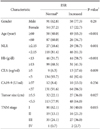
Table 2 shows the relationship between ESR and NLR and clinicopathological characteristics. Significant elevations of ESR were related to old age (more than 60 years) (P<0.001), increased NLR (P=0.001), decreased Hb (P<0.001), increased CEA (P=0.009), increased tumor size (P=0.027) and advanced TNM stage (P=0.015).
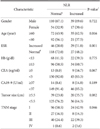
Increased NLR was significantly correlated with old age (more than 60) (P=0.016), increased ESR (P=0.001) and advanced TNM stage (P=0.046) (Table 3).
Survival rate of 1 year, 3 years, 5 years were 86.1%, 68.0%, 65.1%, respectively in the increased ESR group, and 93.4%, 84.0%, 77.4%, respectively in the normal ESR group. There was a statistically significant low survival rate in the increased ESR group (P=0.023) (Fig. 1).
Survival rate of 1 year, 3 years, 5 years were 85.4%, 67.1%, 62.5%, respectively in the increased NLR group and 93.2%, 82.4%, 74.4%, respectively in the normal NLR group. The increased NLR group showed a statistically significant low survival rate (P=0.018) (Fig. 2).
In the univariate analysis, the other clinicopathological variables were associated with shorter overall survival, increased CEA (P<0.001), increased CA19-9 (P<0.001), increased tumor size (P<0.001) and advanced TNM stage (P<0.001).
Multivariate analysis revealed that increased CEA (P=0.033) and TNM stage (P<0.001) were independent prognostic factors for overall survival. Inflammatory parameters (ESR and NLR) did not show significance in the multivariate analysis in our study (Table 4).
Inflammation may occur in over 15% of all malignancies, and the close relationship between inflammation and cancer is widely accepted.2,3 Cancers develop from involved DNA alteration which is caused by phorbor ester, factors released at the site of wounding and inflammation. These factors induce cell proliferation, recruit inflammatory cells and reduce DNA repair. Subversion of cell death and repair programs occurs in chronically inflamed tissues, resulting in DNA replication and proliferation of cells that have lost normal growth control.2
In reverse, the growth and invasion of cancer induces local tissue damage and causes systemic acute phase responses and inflammation. The major role of the acute-phase response is to remove the cause from the body and to restore the initial status; however, if this response persists as it often does in the case of cancer, it contributes to the development of the pathology of the disease.4
Therefore, we hypothesized that the extent of systemic inflammation reflects the progression of cancer and the prognosis of patients with cancer.
The common biomarkers used for systemic inflammation are CRP and ESR. CRP is a sensitive indicator of current disease activity for inflammation. In gastric cancer, an elevated CRP level was reported to be an indicator of disease progression and advanced stage.10,12 Some researchers suggested that CRP could be an independent prognostic factor in patients with gastric cancer.5,10,12
ESR is also used as an indicator of inflammation like CRP. Measurement of ESR is inexpensive and simple to perform. It takes less time for measurement than for CRP.13 Feldman et al.14 reported that ESR and CRP were very highly correlated (P<0.001). High ESR also has been found to correlate with overall poor prognosis for various types of cancer, including Hodgkin's disease, gastric carcinoma, renal cell carcinoma, breast cancer and mucosa associated lymphoid tissue (MALT) lymphoma lymphoma.13,15 According to meta-analysis on renal cell cancer patients, ESR was revealed to be a better indicator of cancer-specific survival than CRP.16 So, we expected the ESR to be a useful marker of inflammation and prognosis in gastric cancer patients.
In our results, elevated ESR, in the univariate analysis, was associated with shorter overall survival (P=0.023) although it could not be proved to be an independent prognostic factor. But significant elevations according to ESR were found related to increased NLR (P=0.001), increased CEA (P=0.009), increased tumor size (P=0.027) and advanced TNM stage (P=0.015). This means that the more advanced the tumor is, the more extensive the inflammation and the higher the ESR level.
High NLR reflects an increased neutrophil and a decreased lymphocyte count. Post-traumatic migration of polymorphonuclear neutrophils (PMN) promotes healing of the injured site, but it can also enhance tumor recurrence. Therefore, preventing postoperative reflux of PMNs may reduce tumor recurrence.17 Previously published studies have shown that lymphopenia is an independent prognostic factor for overall survival in several cancers.18 Therefore, recently there has been increasing evidence that high NLR is a poor prognostic marker in colorectal cancer and gastric cancer.7,18
Also, in our results increased NLR was observed in patients with old age (more than 60 years old) (P=0.016), increased ESR (P=0.001) and advanced TNM stage (P=0.046). The increased NLR group showed a statistically significant low survival rate in univariate analysis (P=0.018). But multivariate analysis failed to prove NLR to be an independent prognostic factor.
This was a retrospective study, which is a limitation of our study. Second, we evaluated inflammatory factors only in the preoperative setting. Third, various factors which affect levels of inflammatory factors such as age, and concomitant infectious or rheumatologic diseases were not controlled for. Although we could not prove that ESR and NLR are independent prognostic factors, we think that a well-designed large-scale study investigating these factors and cancer is needed.
In conclusion, our results indicated that preoperative elevated ESR and NLR could predict the advanced stage of gastric cancer. But this study could not confirmed the prognostic value of ESR and NLR in gastric cancer patients.
Figures and Tables
References
1. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012. 44:11–24.

3. Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000. 248:171–183.

4. Yamashita H, Katai H. Systemic inflammatory response in gastric cancer. World J Surg. 2010. 34:2399–2400.

5. Lukaszewicz-Zając M, Mroczko B, Gryko M, Kędra B, Szmitkowski M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med. 2011. 11:89–96.

6. Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010. 16:5805–5813.

7. Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012. 83:292–299.

8. Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011. 11:489.

9. Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011. 41:510–513.

10. Chang CC, Sun CF, Pai HJ, Wang WK, Hsieh CC, Kuo LM, et al. Preoperative serum C-reactive protein and gastric cancer; clinical-pathological correlation and prognostic significance. Chang Gung Med J. 2010. 33:301–312.
11. Mönig H, Marquardt D, Arendt T, Kloehn S. Limited value of elevated erythrocyte sedimentation rate as an indicator of malignancy. Fam Pract. 2002. 19:436–438.

12. Wang CS, Sun CF. C-reactive protein and malignancy: clinicopathological association and therapeutic implication. Chang Gung Med J. 2009. 32:471–482.
13. Alao OO. Clinical utility of the erythrocyte sedimentation rate. J Clin Med Res. 2010. 2:119–124.
14. Feldman M, Aziz B, Kang GN, Opondo MA, Belz RK, Sellers C. C-reactive protein and erythrocyte sedimentation rate discordance: frequency and causes in adults. Transl Res. 2013. 161:37–43.

15. Todorovic M, Balint B, Jevtic M, Suvajdzic N, Ceric A, Stamatovic D, et al. Primary gastric mucosa associated lymphoid tissue lymphoma: clinical data predicted treatment outcome. World J Gastroenterol. 2008. 14:2388–2393.

16. Wu Y, Fu X, Zhu X, He X, Zou C, Han Y, et al. Prognostic role of systemic inflammatory response in renal cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2011. 137:887–896.

17. van den Tol MP, ten Raa S, van Grevenstein WM, van Rossen ME, Jeekel J, van Eijck CH. The post-surgical inflammatory response provokes enhanced tumour recurrence: a crucial role for neutrophils. Dig Surg. 2007. 24:388–394.

18. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009. 69:5383–5391.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download