Abstract
Purpose
Gastric mucosal neoplastic lesions should have characteristic endoscopic features for successful endoscopic submucosal dissection.
Materials and Methods
Out of the 1,010 endoscopic submucosal dissection, we enrolled 62 patients that had the procedure cancelled. Retrospectively, whether the reasons for cancelling the endoscopic submucosal dissection were consistent with the indications for an endoscopic submucosal dissection were assessed by analyzing the clinical outcomes of the patients that had the surgery.
Results
The cases were divided into two groups; the under-diagnosed group (30 cases; unable to perform an endoscopic submucosal dissection) and the over-diagnosed group (32 cases; unnecessary to perform an endoscopic submucosal dissection), according to the second endoscopic findings, compared with the index conventional white light image. There were six cases in the under-diagnosed group with advanced gastric cancer on the second conventional white light image endoscopy, 17 cases with submucosal invasion on endoscopic ultrasonography findings, 5 cases with a size greater than 3 cm and ulcer, 1 case with diffuse infiltrative endoscopic features, and 1 case with lymph node involvement on computed tomography. A total of 25 patients underwent a gastrectomy to remove a gastric adenocarcinoma. The overall accuracy of the decision to cancel the endoscopic submucosal dissection was 40% (10/25) in the subgroup that had the surgery.
Conclusions
The accuracy of the decision to cancel the endoscopic submucosal dissection, after conventional white light image and endoscopic ultrasonography, was low in this study. Other diagnostic options are needed to arrive at an accurate decision on whether to perform a gastric endoscopic submucosal dissection.
Gastric cancer is one of the leading causes of cancer related deaths in the world, and it is especially common in East Asian countries.(1) One of the major factors associated with improved survival of patients with gastric cancer is early detection. As a result of expansion of both nationwide cancer-screening programs and of private health check-ups, premalignant gastric lesions and early gastric cancer (EGC) are now detected with increasing frequency in Korea.(2) The endoscopic mucosal resection (EMR) has become one of the established treatment procedures for small early gastric cancers without any lymph node involvement and precancerous gastric lesions.(3) EMR is an effective technique for the removal of early GI tract neoplasm. Complication rates associated with the EMR are low. However, lesions over 20 mm cannot be resected in a single piece and piecemeal resection leads to local recurrence rates of up to 15%.(4,5)
A novel technique, the endoscopic submucosal dissection (ESD) has been proposed to guarantee en bloc resection.(6-8) This technique has several advantages: the resected size and shape can be controlled, en bloc resection is possible even with large tumors, and tumors with ulcerative findings are also resectable. In addition, the ESD can provide a precise histological diagnosis and has been associated with a reduced recurrence rate.(5) The ESD can be used for the resection of complex tumors, such as large tumors, ulcerative non-lifting tumors and recurrent tumors.
When performing the ESD, difficulty can arise because the features of the target mucosal lesion might be different in the second endoscopic examination, from the index endoscopy. Erroneous diagnosis by forceps biopsy is a reason to cancel an ESD in addition to disease progression and spontaneous regression.(9,10) If we could enroll the patients with appropriate indication for gastric ESD, the benefit would be avoiding unnecessary surgery and also be avoding unnecessary ESD vice versa.
In this study, the clinical outcomes of cancelled ESD procedures were evaluated. The objective of this study was to determine the accuracy of the decision to cancel an ESD.
Out of 1,010 cases of gastric ESD performed from January 2007 to April 2010, 62 were included in this study. These patients had an ESD planned, but then cancelled. The mean follow-up period was 474.81±285.54 days. The patients with advanced gastric lesions on the initial conventional white light image (cWLI) were excluded. The patients were divided into two groups: Under-diagnosed and over-diagnosed. For example, in cases where the initial diagnosis from a local clinic suggested the need for an ESD, the lesion might have been observed as more advanced than appropriate for an ESD on the second cWLI and endoscopic ultrasonography (EUS), and these cases were defined as under-diagnosed (unable or difficult to perform an ESD). By contrast, if the lesion on the ESD was vague in appearance such as with indistinct margins or as too small or more consistent with gastritis on biopsy, during the second cWLI, these cases were defined as over-diagnosed (unnecessary to perform an ESD). The institutional review board of our center approved this study.
When an ESD was planned, the expanded ESD indications were adopted in cases with adenocarcinoma. The expanded indications for EMR were suggested by Gotoda in 2000. Based on the report, at the National Cancer Center and other groups, the indications for ESD are: (i) non-ulcerated, differentiated-type mucosal carcinomas, regardless of tumor size; and (ii) differentiated-type mucosal carcinoma with an ulcer scar ≤30 mm.(11) If the target lesion did not meet the criteria for the procedure during the second cWLI such as in cases with advanced disease (i.e. submucosal or muscularis propria invasion on EUS, size over 3 cm with ulceration), the procedure was cancelled. In the case of dysplasia, the ESD was cancelled when the lesion to undergo ESD was vague in appearance with indistinct margins, too small, or was confirmed as gastritis on the biopsy from the second endoscopy.
All 62 patients were referred for ESD from local clinics. The endoscopic features and pathology from the local clinics were reviewed. The mean time lag between the first and second endoscopy was 37.65±15.12 days. The second endoscopy for the ESD (GIF-H260, GIF-H180, Olympus, Tokyo, Japan) was performed at our clinic by a single expert (SW Jeon), who has performed more than 1,000 gastric ESDs.
A radial scanning, 20-MHz catheter, probe (UM3R, Olympus), was used by the same physicians in all patients except the patients with advanced gastric cancer (AGC) features on second endoscopy. The probe was passed through the instrument channel of a two channel endoscope (GIF-2T200, Olympus). When the ESD was cancelled, a repeat biopsy (defined as the second diagnosis) and another description of the target lesions was performed.
The reasons for cancelling the ESD were categorized into four groups in the under-diagnosed group: gross AGC features, submucosal invasion in the EUS, size >3 cm with ulceration, and diffuse infiltrative lesion. The consistency between the second diagnosis and the final surgical pathology in the patients that went to surgery was evaluated. In addition, the clinical and endoscopic data were analyzed to assess the relationship between the accuracy and variables that were assumed to be predictive of the accuracy associated with canceling the ESD. In the cases that underwent surgery, the clinical outcomes were reviewed and the accuracy of cancelled cases assessed.
A statistical software package (SPSS ver. 14.0, SPSS Inc., Chicago, IL, USA) was used for the data analysis. For assessment of the association between the accuracy of the decision and the study variables (i.e. category of size, location, endoscopic features, and pathology), the χ2 test was used, and the independent t-test for quantitative variables. P-values less than 0.05 were considered to be significant.
The target lesions were more frequently located in the lower portion of the stomach. Macroscopic types were classified as elevated, flat, and depressed. The depressed type was more common (32 [51.6%]) than the others (15 flat lesions [24.2%] and 15 elevated lesions [24.2%]). The tumor size was categorized into four groups: (<20 mm, 19 [30.6%]; 21~30 mm, 16 [25.8%]; 31~40 mm 14 [22.6%], >40 mm 13 [21.0%]). The pathological diagnosis when referred was 35 low grade dysplasias (LGDs) (56.5%), six high grade dysplasias (HGDs) (9.7%), 19 adenocarcinomas (30.6%) and two atypical cells (3.3%). Twenty-five (40.3%) patients underwent surgical treatment and four (6.5%) patients underwent ESD later (Table 1).
Among the 62 cancelled cases, 30 (19 adenocarcinoma, 5 HGD, 5 LGD, 1 atypical cell in initial diagnosis) were under-diagnosed when referred, and consisted of six cases of gross AGC on the second cWLI (Fig. 1A, B), 18 cases with submucosal invasion on the EUS (Fig. 1C, D), four cases with a size over 3 cm and ulceration, one case with diffuse infiltrative endoscopic features and one case with lymph node involvement on computed tomography (CT) (Fig. 2).
Thirty-two patients were over-diagnosed (1 atypical cells, 1 HGD, and 30 LGD on the initial diagnosis) and their pathology at the time of the second endoscopy was one adenocarcinoma, 17 dysplasias, 11 cases of chronic gastritis and three cases with no suspected mucosal lesions for re-biopsy. Adenocarcinoma identified on the re-biopsied cases underwent ESD later and three cases with dysplasia had ESD later.
In the under-diagnosed group, 25 patients underwent a gastrectomy (subtotal or total, according to the location) with D1-2 dissection. The clinical outcomes of these 25 operated patients are described in Table 2. There was no lymph node involvement on the final surgical pathology.
In the over-diagnosed group, four cases underwent an ESD later. All 4 cases were considered unnecessary to perform ESD due to indistinctive margins on the second endoscopy. The time lag between the second diagnosis and the ESD performed later varied from 20 days to 227 days (Table 3).
Clear margins were obtained by ESD in all four cases and the location of the final lesion on ESD was consistent with the initial location described at the local clinic.
The overall accuracy of the decision to cancel an ESD was 40% (10/25); 33.3% (2/6) in the gross AGC subgroup, 40% (2/5) in the over 3 cm with ulceration subgroup, 46.2% (6/13) in the submucosal invasion by EUS subgroup and none in the one suspected lymph node involvement case on CT (Table 4). Lesion size, endoscopic features, pathology and location of the lesion were not associated with the decision accuracy in the statistical analysis.
The worldwide clinical application of ESD, has allowed more cases of early stage gastric cancer to be treated by endoscopic resection. With the widespread use of endoscopic resection for the treatment of gastric neoplasms, precise staging has become mandatory in order to assess the appropriateness of the procedure for curative treatment.(12-14) With regard to the clinical decision to perform an ESD, it is impossible to assess the precise invasion depth of the forceps biopsy. Thus, EUS is the first-choice imaging modality for determining the depth of invasion.(12,14)
A meta-analysis of 22 studies showed that the accuracy of EUS for T staging in gastric cancer ranges from 65% to 92%.(15) These studies confirmed that EUS is the most accurate staging method for gastric cancer. However, when the studies are limited to early gastric cancer, the accuracy for T staging is only 70~76%.(16,17) A systematic review of 18 studies demonstrated that the sensitivity of EUS in differentiating mucosal cancer from cancer extension beyond the mucosa varied significantly, ranging from 18.2% to 100%.(12) The accuracy of EUS in differentiating between early and AGC is high. However, there are problems in distinguishing T1a from T1b cancer, which is critical in the selection of patients for endoscopic resection of early gastric cancer. The reasons for an incorrect diagnosis with EUS include tumor microinvasion, peritumor inflammation, a distinctly protruding lesion, and oblique scanning.(18) Microinvasion may result in under-staging, as it is difficult to diagnose minimal submucosal invasion. Over-staging may be associated with ulceration, peritumor inflammatory changes, or fibrosis. About 10~30% of early gastric cancers have ulceration with accompanying fibrosis, which is seen as a hypoechoic lesion on EUS, similar to tumor invasion.(18)
In this study of cancelled ESD cases, only six out of 13 cases (operated cases due to cancer invasion suspected in over one third of submucosa by EUS) were accurate according to the final surgical pathology reports. Another 53.8% of cases had intramucosal cancer on the surgical pathology reports, which suggests they underwent avoidable surgery. In this study, large lesions and a high frequency of depressed lesions may have been responsible for lower EUS accuracy in comparison to other studies.
To distinguish cancer invasion from ulcer fibrosis, a method of pattern analysis was introduced. This pattern analysis was based on the fact that ulcer fibrosis always has a fan-shaped spread, while cancer invades in an arched-shaped spread. However, micro-invasion into the ulcer fibrosis does not change the contours of the fan-shaped ulcer fibrosis, so micro-invasion is not detectable by EUS. By using this pattern analysis, it was reported that the diagnostic accuracy for depressed-type EGC with ulceration was 76.1%.(19) Contrast-enhanced EUS was recommended to be another method to improve the accuracy of EUS for lesions with ulcerous changes. If the area of the carcinoma cells was selectively enhanced by intravenous contrast, it might contribute to distinguishing tumor invasions from fibrosis and lead to an accurate diagnosis for lesions with ulcerations.(20) Kida et al.(21) reported that three-dimensional EUS (3D-EUS) provided a practical way to diagnose small invasion of tumors larger than 500 µm with an accuracy of 78.7% when EGC had no ulcerous changes, suggesting that 3D-EUS may be more useful and more accurate for diagnosis. However, even with the use of 3D-EUS, differentiating the minute gastric cancer invasion of ulcer fibrosis from ulcer fibrosis alone has been difficult.
The accuracy of the decision to cancel the procedure due to gross AGC features and a size larger than 3 cm with ulcerations was also low (50%). Perhaps due to the small sample size; however, the endoscopist must be cautious with regard to the decisions on how to manage the AGC lesions. Performing EUS is acceptable in cases that appear to be AGC. Fifty percent of cases with EGC that were large (>3 cm) with ulceration had the potential for complete resection by ESD, in this study. Although a larger study with a randomized controlled design is needed, EUS examination can be carefully performed to assess the possibility of complete resection by ESD in patients with EGC; especially for those where more invasive procedures are contraindicated.
Thirty-two out of 62 cancelled cases were considered to be over-diagnosed on the first endoscopy. For the second diagnosis, well demarcated, definite mucosal lesions for ESD were not observed as in the first diagnosis. In previous studies, the proportion of spontaneous regression of gastric adenomas has been reported to be from 11% up to 74%.(9,22) This wide variability was associated with a diverse proportion of LGD and HGD in the enrolled cases. In 28 cases, lesions that needed endoscopic resection did not reappear during the follow up period. However, demarcation was clearly observed in four cases on the follow up endoscopic examination, and ESD was performed later. One case had an adenocarcinoma on the final ESD pathology.
Narrow band imaging (NBI) has been used as a tool for well demarcation of the lesion and is a video endoscopic imaging technique that enhances the visualization of microstructures and capillaries in the superficial mucosal layer by the use of narrow band filters that change the spectral features of the observed light relative to that of the narrow band filters.(23) Predictions of the histological characteristics of gastric cancer lesions can also be made using NBI and magnifying endoscopy (NBI-ME), which yield very clear images of microvessels on mucosal surfaces.(24,25) One study demonstrated that determining the border of the lesion recognized by differences in capillary structures with NBI-ME is useful and helps with the en bloc EMR of EGC.(26) In addition, autofluorescence imaging (AFI) is one of the newly developed technologies that produce real-time pseudocolor images by detecting natural tissue fluorescence from endogenous fluorophores that are emitted by the excitation of light.(27,28) Several studies have reported that the AFI can reveal minute lesions of the stomach that were not detected by cWLI.(28-31) Therefore, the AFI might also be helpful for the determination of the extent of small gastric lesions before treatments such as EMR/ESD. In this study, determination of the depth of the invasion was the main problem in the under-diagnosed group, whereas a definite demarcation line was an important factor in the over-diagnosed group. These two factors are essential in the determination of the endoscopic resectability of gastric neoplasms. By using only cWLI, it is very difficult to confirm these factors before obtaining surgical pathology. The clinical usefulness of NBI-ME and AFI should be further validated.
The limitations of this study include the following. The study used a retrospective design. Also, 1st endoscopy was performed by various endoscopist in local clinics, but data base on ESD was maintained prospectively by a single endoscopist, thus bias was minimized. Because of the low ESD cancellation rate in this study (6.1%), there was a small sample size (n=62). The third limitation was the use of multiple endoscopy results from multiple local clinics; different imaging procedures might have been performed in the first and second endoscopic procedures with different equipment. The variation in the time interval between the index and second endoscopy might be another limitation of this study. A large prospective randomly controlled trial is needed to further assess the decision-making procedure for ESD.
There are some technical difficulties for achieving successful complete resections and very specific indications are needed to achieve successful complete resections. Other new diagnostic options other than EUS and conventional white light endoscopy are needed to make more accurate clinical decision when considering an ESD.
Figures and Tables
Fig. 1
Two centimeter elevated mucosal lesion was revealed as HGD on the initial diagnosis of a 63-year-old male (A). However, ulceration and a size over 3 cm were observed on the second cWLI 59 days later (B), therefore surgical treatment was performed. EGC was diagnosed on a 3 cm IIa lesion of the antrum in a 75-year-old female (C). However, the lesion already involved over 1/3 of the upper submucosa on the EUS performed 9 days later compared to the initial endoscopy (D). HGD = high grade dysplasia; cWLI = conventional white light image; EGC = early gastric cancer; EUS = endoscopic ultrasonography.
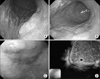
Fig. 2
Clinical outcomes of cancelled ESD cases. Among the 62 cancelled cases, 30 (19 adenocarcinoma, 5 HGD, 5 LGD, 1 atypical cell in initial diagnosis) were under-diagnosed when referred, and included six cases of gross AGC by endoscopic features, 17 cases with submucosal invasion on EUS findings, five cases over 3 cm with ulceration, one case with diffuse infiltrative endoscopic features and one case of lymph node involvement on CT. Twenty-five among the 30 under-diagnosed cases underwent subtotal gastrectomy and none had lymph node involvement. Thirty-two patients were over-diagnosed (1 atypical cell, 1 HGD, 30 LGD in initial diagnosis) and their pathology at the time of the second endoscopy was: 1 adenocarcinoma, 16 adenomas, 11 with chronic gastritis, and four cases with no suspected mucosal lesion for re-biopsy at the second endoscopy. Adenocarcinoma found in re-biopsied cases had ESD later and there were three cases that underwent ESD later with adenomas. ESD = endoscopic submucosal dissection; Adenoca = adenocarcinoma; HGD = high grade dysplasia; LGD = low grade dysplasia; AGC = advanced gastric cancer; EUS = endoscopic ultrasonography; LN = lymph node; CT = computed tomography; F/u = follow-up.

Table 2
Review of 25 cases in the under-diagnosed group that had surgery
ESD = endoscopic submucosal dissection; M = male; F = female; EGC = early gastric cancer; wd = well differentiated; md = moderately differentiated; HGD = high grade dysplasia; LGD = low grade dysplasia; AGC = advanced gastric cancer; sm = submucosa; EUS = endoscopic ultrasonography; LN = lymph node; CT = computed tomography; Adenoca = adenocarcinoma; m = mucosa; mp = muscularis proper; N = no; Y = yes.
References
1. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001. 37:Suppl 8. S4–S66.

2. Nam SY, Choi IJ, Park KW, Kim CG, Lee JY, Kook MC, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009. 21:855–860.

3. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003. 57:567–579.

4. Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, et al. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005. 37:178–182.

5. Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006. 64:877–883.

6. Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006. 41:929–942.

7. Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009. 41:751–757.

8. Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009. 41:746–750.

9. Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004. 36:390–396.

10. Kim YJ, Park JC, Kim JH, Shin SK, Lee SK, Lee YC, et al. Histologic diagnosis based on forceps biopsy is not adequate for determining endoscopic treatment of gastric adenomatous lesions. Endoscopy. 2010. 42:620–626.

11. Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000. 3:219–225.

12. Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol. 2008. 103:1801–1809.

13. Ichikawa T, Kudo M, Matsui S, Okada M, Kitano M. Endoscopic ultrasonography with three miniature probes of different frequency is an accurate diagnostic tool for endoscopic submucosal dissection. Hepatogastroenterology. 2007. 54:325–328.
14. Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008. 14:4011–4019.

15. Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007. 25:2107–2116.

16. Kim JH, Song KS, Youn YH, Lee YC, Cheon JH, Song SY, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007. 66:901–908.

17. Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002. 34:973–978.

18. Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010. 42:705–713.

19. Kida M, Tanabe S, Watanabe M, Kokutou M, Kondou I, Yamada Y, et al. Staging of gastric cancer with endoscopic ultrasonography and endoscopic mucosal resection. Endoscopy. 1998. 30:Suppl 1. A64–A68.

20. Nomura N, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Usefulness of contrast-enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointest Endosc. 1999. 50:555–560.

21. Kida M, Kikuchi H, Ikeda H, Miyazawa S, Iwai T, Araki M, et al. Diagnostic of gastric cancer invasion with three-dimensional endoscopic ultrasonography, especially in cases with "SM1" invasion. Stomach Intest. 2007. 42:88–98.
22. Di Gregorio C, Morandi P, Fante R, De Gaetani C. Gastric dysplasia. A follow-up study. Am J Gastroenterol. 1993. 88:1714–1719.
23. Gono K, Yamazaki K, Doguchi N, Nonami T, Obi T, Yamaguchi M, et al. Endoscopic observation of tissue by narrow band illumination. Opt Rev. 2003. 10:211–215.

24. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004. 36:1080–1084.

25. Sumiyama K, Kaise M, Nakayoshi T, Kato M, Mashiko T, Uchiyama Y, et al. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest Endosc. 2004. 60:79–84.

26. Kadowaki S, Tanaka K, Toyoda H, Kosaka R, Imoto I, Hamada Y, et al. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009. 24:1625–1630.

27. Ohkawa A, Miwa H, Namihisa A, Kobayashi O, Nakaniwa N, Ohkusa T, et al. Diagnostic performance of light-induced fluorescence endoscopy for gastric neoplasms. Endoscopy. 2004. 36:515–521.

28. Uedo N, Iishi H, Tatsuta M, Yamada T, Ogiyama H, Imanaka K, et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005. 62:521–528.

29. Bhunchet E, Shibata T. Proposal for two strategies to prevent remnants of gastric cancers after endoscopic mucosal resections: fluorescein electronic endoscopy and rapid stump diagnosis based on pit patterns. Gastric Cancer. 2004. 7:221–232.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download