Abstract
More than ten years have passed since the sentinel node (SN) concept for gastric cancer surgery was first discussed. Less invasive modified surgical approaches based on the SN concept have already been put into practice for malignant melanoma and breast cancer, however the SN concept is not yet placed in a standard position in gastric cancer surgery even after two multi-institutional prospective clinical trials, the Japan Clinical Oncology Group trial (JCOG0302) and the Japanese Society for Sentinel Node Navigation Surgery (SNNS) trial. What is the problem in the clinical application of the SN concept to gastric cancer surgery? There is no doubt that we need reliable indicator(s) to determine with certainty the absence of metastasis in the lymph nodes in order to avoid unnecessary lymphadenectomy. There are several matters of debate in performing the actual procedure, such as the type of tracer, the site of injection, how to detect and harvest, how to detect metastases of SNs, and learning period. These issues have to be addressed further to establish the most suitable procedure. Novel technologies such as indocyanine green (ICG) fluorescence imaging and one-step nucleic acid amplification (OSNA) may overcome the current difficulties. Once we know what the problems are and how to tackle them, we can pursue the goal.
It is more than 10 years since the sentinel node (SN) concept for gastric cancer surgery was first discussed.(1,2) However, SN for gastric cancer is not yet clinically used universally, although less invasive modified surgical approaches based on the SN concept have already been put into practice for malignant melanoma and breast cancer.
What is the problem in the clinical application of SN in gastric cancer surgery? As a member of the first group to use SN biopsy with indocyanine green (ICG) in gastric cancer surgery,(2) I review here the current status of SN for gastric cancer surgery and discuss these problems.
Gastrectomy with regional lymphadenectomy is indicated for patients with gastric cancer and clinically suspicious lymph node (LN) metastases. The procedure is associated with a satisfactory long-term outcome. This "standard procedure" is usually conducted even when the primary tumor is small. However, there is controversy regarding the application of this procedure for patients with T1 gastric cancer because most of these patients are free of nodal metastases. In terms of the results, it appears that lymphadenectomy is being conducted at far higher rate than necessary in these patients. Thus, we need a reliable indicator that confirms the absence of LN metastases with high accuracy, in order to exclude lymphadenectomy from the surgical procedure used for patients with T1 gastric cancer and preserve a larger volume of the stomach without jeopardizing long-term outcome.
The SN is defined as a LN that directly drains a specific cancer. The concept of SN is based on the notion that non-SNs are unlikely to contain cancer cells if the SN is cancer cell-free. In 1992, Morton et al.(3) demonstrated the concept of SN in a clinical study involving patients with malignant melanoma. Since then, the SN technique has been applied to the surgical management of a variety of cancers to avoid unnecessary lymphadenectomy.(4,5)
With regard to gastric cancer, two Japanese studies were reported around the outset of the 21st century. Hiratsuka et al.(2) reported that SN biopsy using ICG can be performed with a high success rate, and that the SN status can predict the LN status with a high degree of accuracy. Kitagawa et al.(1) reported their preliminary data using intraoperative radiation technique and a gamma probe.
After the above two pioneering works in this area, several single institutional studies supported the validity of the SN concept for gastric cancer. In most of these studies, patients had T1 gastric cancer, and thus only a small number of patients reported in those studies had LN metastases. Moreover, the SN biopsy protocol varies according to the surgeon. Therefore, not only single institutional studies with small number in different ways, but also studies with large number in a unified way have been conducted to evaluate the feasibility and clinical application of this technique. Under these circumstances, two multi-institutional prospective clinical trials; the Japan Clinical Oncology Group trial (JCOG0302) and the Japanese Society for Sentinel Node Navigation Surgery (SNNS) trial, were conducted in Japan. The design and results of the two studies showed some rather large differences, as summarized in Table 1.
The multicenter clinical trial, JCOG0302 (GCSSG-SNB, UMIN-CTR ID: C000000059), was designed to evaluate the feasibility and accuracy of diagnosis using SN biopsy in T1 gastric cancer (Fig. 1). In other words, the feasibility of excluding lymphadenectomy during surgery for T1 gastric cancer was evaluated in patients with green nodes, representing SNs detected by ICG, which were considered cancer cell-free, by using intraoperative histopathological examination of frozen sections with hematoxylin-eosin (H&E) staining. The primary endpoint was the false negative rate (number of patients with an intraoperative negative SN biopsy/number of patients confirmed later to have LN metastases). Patients with only intraoperative SN-positive biopsy were defined as "false positive" in the JCOG0302 trial. Caution should be exercised because patients with intraoperative SN-negative biopsy but SN-positive histopathological examination of paraffin sections were defined as false negative in the trial although such SN biopsy was considered feasible based on the SN concept. The trial started in 2004, however recruitment of patients was terminated midway before the goal because of the unexpected high false negative rate. Our pilot study adopted multi-planes for detection of metastases,(2) however, in the multicenter trial setting, only one plane of largest dimension of frozen section was adopted in the trial for convenience. The planned learning period of 5 patients in each institution is probably an underestimation because the true number is considered to be 30 patients at present, as determined by the Japanese Society for SNNS. That is to say, the JCOG0302 was a trial to evaluate the feasibility of the current clinical setting, including several issues on the SN concept as well as intraoperative histopathological examination using frozen sections and learning period. Detailed analysis of the false negative cases indicated that these issues adversely affected the outcome than expected (unpublished data).
Another multicenter clinical trial was conducted by the Japanese Society for SNNS to evaluate the diagnostic accuracy of SNNS based on radio-guided method for LN metastases. The primary endpoint was the sensitivity of detection of metastases (number of patients with confirmed diagnosis of positive SN biopsy/number of patients with confirmed diagnosis of LN metastases). The SNNS trial differed from the JCOG0302 trial in that the entry criteria included both T1 and T2 gastric cancer, SN biopsy was performed by the dual tracer method (radioactivity was a mandatory requirement), SNs could be harvested in both surgical field and background table after gastrectomy, histopathological examination was performed on both frozen and paraffin sections, and only 12 well-experienced hospitals with more than 30 patients for learning period were included. The aim of the trial was to evaluate the SN concept in gastric cancer surgery based on the premise of substantial experience. The trial setting placed less emphasis on the accuracy of intraoperative histopathological examination of frozen sections and learning period, than the JCOG0302 trial. However, further steps are theoretically required for clinical application because detection of SN was unregulated before gastrectomy with lymphadenectomy.
There are several matters of debate in performing the actual procedures, such as the type of tracer, the site of injection, how to detect and harvest, and how to detect metastatic SNs. These issues have to be addressed further to establish the best procedure.
The dye-guided method is safe, convenient, and cost-effective, whereas legal considerations and costs of radioactive substances limit the probe-guided method in general hospitals.(6,7) However, the dye-guided method has certain limitations, such as loss of visibility in dense fat and rapid transit of the dye, and these limitations are more critical in laparoscopic surgery. Subgroup analyses of meta-analyses showed that the combination of dye and radioactive colloid detection substances is better for detection.(8,9) Either way, adequate training is required as is evident.(10-12)
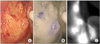
ICG is a popular diagnostic reagent approved clinically,(13) and allergic reactions to ICG are fewer than those to blue dye such as isosulfan blue (Lymphazurin™).(14) As a tracer for SN biopsy, the injected ICG binds rapidly to albumin and is carried more specifically through the lymphatic vessels than indigo carmine or Evans blue.(15) Some ICG-based novel techniques such as infrared ray electronic endoscopy(16,17) and ICG fluorescence imaging(18-20) have been reported as convenient and reliable detection methods for clinical application of this technique using ICG dye (Fig. 2). Such newcomers could improve over time the cumbersome procedure of combined tracers.
Theoretically, appropriate injection of tracer is essential for detection of SNs. In other words, the site of injection (submucosal or subserosal) does not matter if all-round of the tumor is suffused by the injected tracer. Some have argued that the injection site of the tracer does not have to be limited to the submucosa.(21,22) Our experience also underwrites this argument (unpublished data). I guess that many surgeons mix up injection from luminal outside (so-called "subserosal injection") with injection only into the subserosal tight space, which is unrealistic.
The submucosal injection appears to be more reasonable than the subserosal injection in case of luminal organs probably due to accessibility by endoscopy. However, a point to remember is that the indications for SN should include T1 gastric cancer patients with ulceration, who are not suitable for endoscopic resection. Appropriate injection of the tracer in such patients using endoscopy is difficult. On the other hand, subserosal injection into tumors on the lesser curve of the stomach is not easy and that is considered to be impractical in laparoscopic surgery.
The main methods used to detect and harvest SNs are nodes pickup biopsy(2) and lymphatic basin dissection.(23) Some have also proposed station instead of basin dissection.(24) Different from the pickup biopsy of a couple of nodes, basin dissection is a regional lymphadenectomy of one to two basins out of 5 basins of the stomach. It is obvious that finding true SNs is more difficult in the pickup method. Lee et al.(25) reported that a small number of SNs was found to be associated with false-negativity. However, the notion that basin dissection alone is sufficient for patients with T1 gastric cancer who are not suitable for endoscopic resection, is in conflict with the golden standard of gastrectomy with lymphadenectomy. Adversely, if the above notion sticks to facts, it becomes unnecessary to detect and diagnose SNs because lymphatic flow only can guide us to the exact basin to dissect.
In principle, however, basin dissection is in conflict with the SN concept, i.e., elimination of LN dissection. I believe we should stick to the main goal of the SN concept.
As already stated, a reasonable learning period is about 30 patients, as concluded from the survey conducted the Japanese Society for SNNS. Lee et al.(26) reported that the learning period is 26. The learning period of just 5 patients for each institution (but not surgeon) advocated by the JCOG0302 trial is presumably an underestimation, and undoubtedly had a negative effect on the results of the trial.
For clinical application of SN concept in gastric cancer surgery to avoid unnecessary lymphadenectomy, negative diagnosis of metastatic LN should be conducted intraoperatively before gastrectomy. Consequently, highly accurate intraoperative diagnostic methods are in great demand. Both the JCOG0302 trial and the SNNS trial indicated that intraoperative histopathological examination in one plane of largest dimension by frozen section with H&E staining is not highly accurate (unpublished data).
Recently, a novel semi-automated molecular-based rapid diagnostic method for LN metastases has been developed in breast cancer using one-step nucleic acid amplification (OSNA), which requires approximately 30 minutes for final diagnosis.(27) A prospective clinical trial of OSNA in four institutions including ours indicated that the method is feasible for intraoperative detection of LN metastases in patients with gastric cancer (paper in submission). Such new technology could overcome the difficulty of securing highly accurate intraoperative diagnosis.
Figures and Tables
Fig. 1
Trial scheme of the JCOG0302. Intraoperative detection of metastases in one plane of largest dimension of green nodes as SNs was performed using frozen sections and hematoxylin-eosin staining. Gastrectomy with lymphadenectomy was performed after SN biopsy. JCOG0302 = Japan Clinical Oncology Group trial; ICG = indocyanine green; SN = sentinel node.

Fig. 2
ICG-based novel techniques. Infrared imaging (B) allows easy visualization of the LNs, which are otherwise hardly recognized by ICG green color alone (A). The ICG fluorescence imaging (C) also allows separate visualization of LNs. Quotation from reference number 18 cited with minor alteration. ICG = indocyanine green; LN = lymph node.
References
1. Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, Watanabe M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am. 2000. 80:1799–1809.

2. Hiratsuka M, Miyashiro I, Ishikawa O, Furukawa H, Motomura K, Ohigashi H, et al. Application of sentinel node biopsy to gastric cancer surgery. Surgery. 2001. 129:335–340.

3. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992. 127:392–399.

4. Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993. 2:335–339.

5. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994. 220:391–398.

6. Bostick P, Essner R, Glass E, Kelley M, Sarantou T, Foshag LJ, et al. Comparison of blue dye and probe-assisted intraoperative lymphatic mapping in melanoma to identify sentinel nodes in 100 lymphatic basins. Arch Surg. 1999. 134:43–49.

7. Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997. 15:2345–2350.

8. Lips DJ, Schutte HW, van der Linden RL, Dassen AE, Voogd AC, Bosscha K. Sentinel lymph node biopsy to direct treatment in gastric cancer. A systematic review of the literature. Eur J Surg Oncol. 2011. 37:655–661.

9. Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2011. [Epub ahead of print].

10. Morrow M, Rademaker AW, Bethke KP, Talamonti MS, Dawes LG, Clauson J, et al. Learning sentinel node biopsy: results of a prospective randomized trial of two techniques. Surgery. 1999. 126:714–720.

11. Sanidas EE, de Bree E, Tsiftsis DD. How many cases are enough for accreditation in sentinel lymph node biopsy in breast cancer? Am J Surg. 2003. 185:202–210.

12. Cox CE, Salud CJ, Cantor A, Bass SS, Peltz ES, Ebert MD, et al. Learning curves for breast cancer sentinel lymph node mapping based on surgical volume analysis. J Am Coll Surg. 2001. 193:593–600.

13. Caesar J, Shaldon S, ChiandussiI L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961. 21:43–57.
14. Cimmino VM, Brown AC, Szocik JF, Pass HA, Moline S, De SK, et al. Allergic reactions to isosulfan blue during sentinel node biopsy--a common event. Surgery. 2001. 130:439–442.

15. Takayama S, Furuhama K, Ohura K, Onodera T, Akimoto T. Experimental studies on the usefulness of indocyanine green (ICG) as a lymphatic vital dye. Oyo Yakuri. Pharmacometrics. 1980. 19:603–614. (in Japanese).
16. Nimura H, Narimiya N, Mitsumori N, Yamazaki Y, Yanaga K, Urashima M. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg. 2004. 91:575–579.

17. Ishikawa K, Yasuda K, Shiromizu A, Etoh T, Shiraishi N, Kitano S. Laparoscopic sentinel node navigation achieved by infrared ray electronic endoscopy system in patients with gastric cancer. Surg Endosc. 2007. 21:1131–1134.

18. Miyashiro I, Miyoshi N, Hiratsuka M, Kishi K, Yamada T, Ohue M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008. 15:1640–1643.

19. Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008. 25:103–108.

20. Miyashiro I, Kishi K, Yano M, Tanaka K, Motoori M, Ohue M, et al. Laparoscopic detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging. Surg Endosc. 2011. 25:1672–1676.

21. Lee JH, Ryu KW, Kim CG, Kim SK, Choi IJ, Kim YW, et al. Comparative study of the subserosal versus submucosal dye injection method for sentinel node biopsy in gastric cancer. Eur J Surg Oncol. 2005. 31:965–968.

22. Yaguchi Y, Ichikura T, Ono S, Tsujimoto H, Sugasawa H, Sakamoto N, et al. How should tracers be injected to detect for sentinel nodes in gastric cancer--submucosally from inside or subserosally from outside of the stomach? J Exp Clin Cancer Res. 2008. 27:79.
23. Miwa K, Kinami S, Taniguchi K, Fushida S, Fujimura T, Nonomura A. Mapping sentinel nodes in patients with early-stage gastric carcinoma. Br J Surg. 2003. 90:178–182.

24. Ichikura T, Chochi K, Sugasawa H, Yaguchi Y, Sakamoto N, Takahata R, et al. Individualized surgery for early gastric cancer guided by sentinel node biopsy. Surgery. 2006. 139:501–507.

25. Lee JH, Ryu KW, Nam BH, Kook MC, Cho SJ, Lee JY, et al. Factors associated with detection failure and false-negative sentinel node biopsy findings in gastric cancer: results of prospective single center trials. J Surg Oncol. 2009. 99:137–142.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download