Abstract
Purpose
We conducted this study to study the clinical correlation between the characteristics of gastric cancer and serum selenium and zinc levels.
Materials and Methods
The following data were measured in the baseline serum selenium and zinc levels of 74 patients with curative gastrectomy subsequent to confirmed gastric cancer, from March 2005 to August 2012.
Results
Among the 74 gastric cancer patients, 53 patients were male. Mean serum selenium and zinc levels were 118.7±33.1 ug/L and 72.2±24.3 ug/dl, respectively. Seven patients (9.5%) showed lower selenium level, and 33 patients (44.6%) showed lower zinc level. Serum Selenium level was 99.1±31.8 ug/L in cardia cancer group (10 cases) and 121.8±32.4 ug/L in non-cardia cancer group (64 cases)(P=0.044). According to tumor gross type, zinc level was 78.7±29.6 ug/dl in early gastric cancer (33) and 66.9±17.8 ug/dl in advanced gastric cancer (41) (P=0.064).
Selenium (Se) is an essential dietary component for humans, and there is increasing evidence for the efficacy of certain forms of Se as an anticancer nutrient.(1,2) In blood, Se is found in plasma as well as in the cellular compartment, where concentrations are higher and concentrations are highest in tissues like liver, kidneys, and spleen.(3) More than 20 selenoproteins or protein subunits are known to date. The best-known is glutathione peroxidase which reduce peroxides like H2O2 or lipid peroxides and are also involved in transcription control of immune system cells.(4,5) Nutritional deficiency of Se has been associated with Keshan disease, which has been reported only from China. Keshan disease is a dilated cardiomyopathy, which caused a high number of fatalities in a particular area in China where Se concentration in the soil is very low.(6)
Zinc (Zn) is also trace element found in blood. Most of it is contained in bones, skin, and hair (~70%), with the remainder mainly in liver, kidneys, and muscle. In plasma, one-third of the Zn is tightly bound to α2-macroglobulin, the remainder more loosely to albumin. Zn is a structural element of copper-Zn-superoxide dismutase which is intracellularly distributed in red blood cell and may act as a scavenger of active oxygen. By now, at least 50 Zn-dependent enzymatic reactions are known in humans. It influences the proliferation rate of T-lymphocytes (via DNA synthesis and/or interleukin-2), which pathways explain the reduced activity levels of T-helper and NK-cells (natural killer cells) during Zn deficiency.(3,7)
There are many epidemiological studies concerning serum Se and Zn level and/or Se supplement and cancer risks. Most of them are lung cancer, breast cancer, prostatic cancer, and colorectal cancers.(8) So far, there are few studies concerning the clinicopathological feature of gastric cancer and serum Se and Zn level.(9) Also up to this point, the studies about gastric cancer was mainly focused on the occurrence of gastric cancer, their surgical treatment, chemotherapy and prognosis. As we know over recent decades, major progress has occurred in our understanding of increasing focus on improved nutrition in surgical care. Before we make good nutritional support, we have to know the exact status and function related such trace element in gastric cancer patient. And also more study is necessary to know the long-term effect of such micro-nutrient and influence on gastric cancer prognosis. We performed this study to evaluate the correlation between the clinicopathologic feature of gastric cancer and nutritional indicators (hemoglobin [Hb], albumin, age, body mass index [BMI], etc.) and serum Se and Zn level.
We retrospectively reviewed 74 gastric cancer patients whom we performed curative gastrectomy and measured baseline serum Se and Zn levels during from March 2005 to August 2012. The following data was collected; gender, age, BMI, tumor location, tumor gross type, hemoglobin, albumin, Se, Zn, perioperative morbidities and postoperative admission day. Serums of the patients were sent to green cross laboratory, and they measured serum Se level by atomic absorption spectrometry technique and serum Zn level by inductive coupled plasma mass spectrometry technique. Normal serum range of the Se and Zn levels are 76~205 ug/L and 70~121 ug/dl, respectively. This study was approved by the Seoul Medical Center Institutional Review Board (2012-038).
Statistical analysis was performed in IBM SPSS for Windows ver. 20 (IBM Co., Armonk, NY, USA). Categorical variables were summarized using frequencies and percentages; continuous variables were summarized using sample size, mean, median, standard deviation, minimum and maximum. An association between categorical group (age, sex, BMI, Hb, albumin, tumor type and location) and Se and Zn was calculated using independent two sample t-test and one-way ANOVA according to number of categorical groups. Chi-sqaure test was used to compare odd ratio between two categorical groups (divided by Socioeconomic status and morbidity). For adjustment of age, body weight, sex, we used general linear model for continuous dependent variables (Se and Zn) (Table 1). For comparison of Se and Zn according to interaction of two groups, tumor type and location, we used two way ANOVA (Fig. 1). Significance was declared at a two-sided 0.05 level, unless otherwise specified.
Among 74 gastric cancer patients, 53 patients were male. Mean age was 65.3±10.5 years (range, 30 to 88 years). Mean serum Se and Zn levels were 118.7±33.1 ug/L and 72.2±24.3 ug/dl, respectively. Seven patients (9.5%) showed lower Se level and 33 patients (44.6%) showed lower Zn level. There was one postoperative mortality and overall morbidity rate was 39.2% (29 cases) (Table 2).
Serum Se and Zn level were slightly lower in male patients group, older group (age≥70 years), underweight group (BMI<20), anemia group (Hb≤9) and hypoalbuminemia group (albumin≤3.0) (P>0.05) (Table 3).
Serum Se level was significantly lower in cardia cancer group (10 cases, 99.1±31.8 ug/L) than non-cardia cancer group (64 cases, 121.8±32.4 ug/L) (P=0.044) According to tumor gross type, Zn level was lower in the early gastric cancer (EGC) patients group (33 cases, 78.7±29.6 ug/dl) than the advanced gastric cancer (AGC) patients group (41 cases, 66.9±17.8 ug/dl) (P=0.064) (Table 1).
National health insurance (NHI) hired group (46 cases) was significantly higher serum Se level (127.6±34.4 ug/L) than medical aid (MA) group (28 cases, 104.1±25.1 ug/L) (P=0.0024) (Table 4).
When we compared two groups divided by morbidity present group or not, serum albumin and Zn level were significantly lower in morbidity present group (albumin; 3.66 g/dl vs. 3.98 g/dl, P=0.029, Zn; 64.8 ug/dl vs. 76.9 ug/dl) (P=0.037). Hemoglobin and Se level were insignificantly lower in morbidity present group. Postoperative admission day was significantly longer in morbidity present group (29.3±15.5 days) than absent group (12.7±4.4 days) (P<0.001) (Table 5).
Inhibitory effects of Se on carcinogenic process in animal experiments and inverse relationship with cancer incidence and mortality in many ecologic and epidemiologic studies have been well demonstrated.(2,10-12) In several case-control studies, lower serum levels of Zn in lung, esophageal and other cancer patients were observed compared to normal control.(13) And also it is suspected that there is interactive effects between Zn and retinol in inhibiting cancer.(14) It is known that toenail Se level examination acts as an indicator for long-term dietary Se exposure.(15) But plasma Se level is ease to check and also have correlation with Se intake(16) so, in many studies they use blood examination. Overall, the evidence currently available appears to support an inverse association between Se exposure and prostate cancer risk, and possibly also a reduction in risk with respect to lung cancer. But, the results of studies of the association between Se and gastric cancer risk have varied somewhat, although most have yielded point estimates at or below unity.(8) Most of the previous study was done by experimental or epidemiological observation with this trace elements. The significance of this clinical study is that we analyzed the correlation of clinicopathological feature of gastric cancer and serum Se and Zn level.
Kabuto et al.(9) reported that there was little evidence of increased risks of stomach cancer in their cohort study of atomic bomb survivors at Hiroshima and Nagasaki, but risks of lung cancer were highest in the lowest quartiles for both Se (odds ratio [OR]=1.8) and Zn (OR=1.3). In that study, they found that mean Se concentrations showed a decline with age, were higher in male than in females. We also found that serum level of Se was decreased in older than 70 group (120.5±35.6 ug/L) than younger than 60 group (125±32.5) insignificantly (P=0.36).
Rhee et al.(17) reported that the mean Se concentration in cancer patient group (stomach cancer 56 cases, colon cancer 17 cases) was 143.6±10.8 ug/L and for the normal controls (283 cases) serum Se level was 167.0±14.5 ug/L (P<0.001). In the multiple logistic regression analysis, the blood Se concentration (coefficient -0.1184, OR 0.888, 95% confidence interval: 0.860~0.918), age, BMI were significant variables for risk of cancer. Song et al.(18) also reported that the mean serum Se level of the prostate cancer patients (112 cases) was 110.2 ug/L and mean serum Se level of control group (278 cases) was 116.8 ug/L (P=0.250). The serum concentration of Se depends on Se concentration in the soil and daily Se intake. So the mean level of the population will have difference within the country, because of the difference in the concentration of Se in the soil.(6) Probably the place of normal control population of Rhee et al.'s study(17) was Gyeonsang provinces area and the Song et al.'s study(18) was Seoul area. The difference of the two studies' normal controls' Se level might be influenced by the location of the normal population.
We also found that underweight patients (BMI<20, 16 cases) had lower serum Se level compared to normal weight patients (20≤BMI<25, 42 cases) insignificantly (P=0.09). So, if the patient have severe weight loss or underweight condition and is older age group, checking the serum Se level is advisable and replacement therapy may be necessary.
According to Mark et al.(19)'s prospective randomized nutritional intervention trial, they found that participants who received Se, β-carotene, and vitamin E had significantly lower cancer mortality rates than those who did not and significant inverse associations of serum Se levels with the incidence of esophageal and gastric cardia cancers. In that study, they investigated cancer incidence divided esophagus, gastric cardia and non-cardia group. The serum levels of Se in cardia cancer group were 68.4±0.25 ug/L and esophageal cancer groups were 69.6±0.19 ug/L. They were significantly lower serum Se concentration than control subjects (P<0.001). The subjects with gastric non-cardia cancers (73.3±0.66 ug/L) had statistically not significant higher mean Se values than the control subjects (72.3±0.59 ug/L) (P=0.29). Although most of other studies have investigated the association of gastric cancer overall with serum Se level, but this was probably the only prospective study evaluating the association of serum Se levels with the occurance of esophageal cancer or gastric cancers by subsite.
We also found that the serum Se level in the cardia group (99.1±31.8 ug/L) was significantly lower than in the non-cardia group (121.8±32.4 ug/L) (P=0.044). But there was no correlation between the serum level of Zn and tumor location. As we divided the patients according to the tumor gross type (EGC vs. AGC), serum Se level of EGC group (121.2±32.1 ug/L) was insignificantly higher than AGC group (116.7±34.1 ug/L)(P=0.567). Serum Zn level of AGC group (66.9±17.8 ug/dl) was lower than in EGC group (78.7±29.6 ug/dl) (P=0.064).
We divided the patient into two groups according to NHI hired status to know the influence of socioeconomic status on serum Se and Zn level. NHI hired group vs. MA group. As we expected that MA group will have poor nutritional profiles, serum Se level of MA group was 104.1±25.1 ug/L and it was lower than NHI group's level (127.6±34.4 ug/L) (P=0.0024). It is partially considered to have correlation with difference in the ratio of EGC and AGC in both groups. In NHI group, 50% of the patients were EGC. But, only 35.7% of the patients were EGC in MA group. But, there was no difference in serum hemoglobin, albumin and Zn level between two groups.
Zn deficiency can result in an inhibition of cellular proliferation and deficient granulation tissue formation and healing.(20) In our study, overall postoperative morbidity happened in 29 cases (39.2%, one case of post-operative mortality included). Compared with morbidity absent group, serum albumin and Zn level were significantly lower in morbidity present group (P=0.029, 0.037, respectively). Most importantly, the morbidity rate in the NHI group was 23.9%, but in the MA group, it was significantly high (64.3%) (P<0.001) (Table 5).
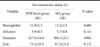
When we compared serum Se and Zn level according to tumor gross type (EGC vs. AGC) and tumor location(cardia vs. non-cardia) by two way ANOVA, Se has significant difference (P=0.021) between the groups but, not in Zn. Especially, it showed the most significant difference between cardia EGC and cardia AGC groups (P=0.009) (Fig. 1).
Serum Zn level was slightly lower in man, older than 70s, lower BMI (<20), hypoalbuminemia, and AGC patient. When we manage such patient, it is desirable to check such trace elements' level preoperatively and if there is trace element deficiency, replacement therapy may be necessary. But, pharmacologic overdosing with Zn does not accelerate wound healing and can have detrimental effects.(21)
Serum Se level was highly correlated with location of gastric cancer. Low serum Se level is one kind of many nutritional problems that happen in high body AGC and may influence the prognosis of the patient. Serum Zn level was slightly lower in AGC. It is necessary to check such trace elements in high body AGC. We need more prospective studies to evaluate the correlation between serum Se and Zn levels and perioperative morbidities and cancer prognosis and benefit of such elements replacement therapy.
Figures and Tables
Fig. 1
Comparison of (A) selenium and (B) zinc level with two clinical feature of gastric cancer (mean). EGC = early gastric cancer; AGC = advanced gastric cancer. *P=0.009 (tow extreme value of AGC and EGC in Cardia group).

References
1. Zeng H, Combs GF Jr. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008. 19:1–7.

2. Combs GF Jr. Current evidence and research needs to support a health claim for selenium and cancer prevention. J Nutr. 2005. 135:343–347.

3. Biesalski HK, Grimm P. Pocket Atlas of Nutrition. 2005. 3rd ed. Stuttgart: Georg Thieme Verlag.
4. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973. 179:588–590.

6. Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda). 2006. 21:307–315.

7. Fukuzawa K. Kondo M, editor. Biological defense systems in oxygen stress and new antioxidant drugs based on these systems. Free Radicals in Clinical Medicine. 1988. Vol. 3. Tokyo: Nihon-Igakukan.
8. Navarro Silvera SA, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control. 2007. 18:7–27.

9. Kabuto M, Imai H, Yonezawa C, Neriishi K, Akiba S, Kato H, et al. Prediagnostic serum selenium and zinc levels and subsequent risk of lung and stomach cancer in Japan. Cancer Epidemiol Biomarkers Prev. 1994. 3:465–469.
10. Birt DF. Update on the effects of vitamins A, C, and E and selenium on carcinogenesis. Proc Soc Exp Biol Med. 1986. 183:311–320.

11. Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991. 51:595–600.
12. Shamberger RJ, Tytko SA, Willis CE. Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Arch Environ Health. 1976. 31:231–235.
13. Atukorala S, Basu TK, Dickerson JW, Donaldson D, Sakula A. Vitamin A, zinc and lung cancer. Br J Cancer. 1979. 40:927–931.

14. Prasad AS. Clinical, biochemical and nutritional spectrum of zinc deficiency in human subjects: an update. Nutr Rev. 1983. 41:197–208.

15. Morris JS, Stampfer MJ, Willett WC. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res. 1983. 5:529–537.

16. Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996. 7:384–390.

17. Rhee JK, Chung JH, Sakong J, Kang PS, Kim CY, Lee KS. Association between cancer and selenium concentration in blood and toenails. Yeungnam Univ J Med. 1992. 9:29–43.

18. Song SH, Song K, Lee SB, Kim CS. The serum selenium level in Korean men and its association with age and prostate cancer. Korean J Urol. 2006. 47:150–153.

19. Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000. 92:1753–1763.

20. Fernandez-Madrid F, Prasad AS, Oberleas D. Effect of zinc deficiency on nucleic acids, collagen, and noncollagenous protein of the connective tissue. J Lab Clin Med. 1973. 82:951–961.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download