Abstract
Purpose
The aim of this study is to evaluate the feasibility and safety of cardia preserving proximal gastrectomy, in early gastric cancer of the upper third.
Materials and Methods
A total of 10 patients were diagnosed with early gastric cancer of the upper third through endoscopic biopsy. The operation time, length of resection free margin, number of resected lymph nodes and postoperative complications, gastrointestinal symptoms, nutritional status, anastomotic stricture, and recurrence were examined.
Results
There were 5 males and 5 females. The mean age was 56.5±0.5 years. The mean operation time was 188.5±0.5 minutes (laparoscopic operation was 270 minutes). Nine patients were T1 stage (T2 : 1), and N stage was all N0. The mean number of resected lymph nodes was 25.2±0.5. The length of proximal resection free margin was 3.1±0.1 cm and distal was 3.7±0.1 cm. Early complications were surgical site infection (1), bleeding (1), and gastro-esophageal reflux disease (1) (this symptom was improved with medication). Late complications were dyspepsia (3) (this symptom was improved without any treatment), and others were nonspecific results of endoscopy or symptom.
Recently, detection of early gastric cancer has increased due to the development of diagnostic technologies and expansion of medical check-ups, as well as the expansion of the upper gastric cancer portion.(1) The incidence rates of gastric cancer in Korea has decreased, but cancer is one of the major causes of death and the Statistics Korea released in 2011 that the gastric cancer was the third highest cause of death among the Korean population.(2) Under such circumstances, the central government has invested a great deal to the efforts of early diagnosis and treatment, and expanded the attentions to upgrading the quality of lives after the surgery. However, unlike other parts, total gastrectomy is performed on the upper gastric cancer due to many complications from partial gastrectomy, and the Japanese gastric cancer treatment guideline recommends total gastrectomy as the standard treatment. Many operation methods have been introduced to prevent gastroesophageal reflux or anastomotic stricture after total gastrectomy with high interests in quality of lives recently. Still, there have been debates on the scope of excision, radical cure and the survival rates, complications and quality of lives after the operation.(3,4) In particular, total or proximal partial gastrectomy is performed whether safe excision and remaining stomach are secured for the upper early gastric cancer and total gastrectomy may cause to lose functions, including storing, crushing and mixing foods, exocrine and endocrine, and nutrition.(5,6) Meanwhile, the paper by An et al.(3) published in 2008 about comparing the total and the proximal partial gastrectomies showed that the two methods had similar results, but the former has 1.8% and 6.9% complication rates of reflux esophagitis and anastomotic stricture, respectively, comparing 29.2% and 38.2% for the proximal partial gastrectomy with significance. It is inferred to lose lower esophageal sphincter (LES) and phrenoesophageal ligament (PEL), favoring total gastrectomy more.(3)
Against this backdrop, the authors performed cardia preserving proximal gastrectomy (CPPG) performed for upper early gastric cancer, satisfying some limited conditions to maintain the nutritional advantages of partial gastrectomy, while preventing reflux esophagitis and anastomotic stricture by preserving the PEL and cardia with LES as an operation method to complement general proximal partial gastrectomy.
The study was performed for 10 patients who had consecutive CPPG at Chosun University Hospital in early gastric cancer diagnosed under endoscopic biopsy, from October 2006 to April 2011.
Computed tomography (CT) was performed to check hypertrophy in perigastric lymph nodes for precise evaluation among the patients diagnosed with early gastric cancer on the upper part, before the surgery under endoscopic results.
The patients under the CPPG were limited to those who were diagnosed as early gastric cancer, based on the endoscopic results with no lymph node hypertrophy on the CT located on the top 4 cm from the esophago-gastric junction (E-G junction) (Fig. 1). This is because the cardia with 2 cm size from E-G junction shall be preserved, and the excision shall be performed by securing the proximal free resection margin of more than 2 cm. After omentectomy, to preserve the right gastroepiploic artery, we checked the 6th lymph node with fingers without enbloc dissection. It was confirmed that no metastasis occurred by performing frozen biopsy for the dissected lymph nodes and the greater omentum was dissected toward the spleen to remove all the 4th lymph nodes and the gastroepiploic vessels were dissected on 2/3 from the top to the bottom of the greater curvature, to remove the greater omentum, and divided the short gastric vessels to separate the fundus ventriculi from the spleen.
The 12th lymph nodes were dissected, and the lesser curvature and the 5th and the 3rd lymph nodes were dissected toward from the origin of the right gastric artery to the esophagus. Then, the metastasis was confirmed with frozen biopsy of selectively dissected lymph nodes to preserve the right gastric vessels for the 5th lymph nodes with the same method as the 6th lymph nodes. The branch of gastric vessels in the 2/3 of the lesser curvature was divided from the top, and was removed with the lesser omentum.
The abdominal esophagus was not widely dissected to avoid damage of PEL on the diaphragm, and the palpated lymph nodes without complete separation, like the 5th, and the 6th lymph nodes were excised on perigastric lymph nodes (1st and 2nd lymph nodes) in the cardia. After confirming that the metastasis was not discovered through a frozen biopsy, an excision was performed about 2 cm from the bottom to the top on E-G junction and securing the proximal free resection margin was confirmed through a frozen biopsy. The CPPG was not intended to proceed any more in case of selectively separating the abovementioned lymph nodes and suspicious of metastasis on the lymph nodes. Then, the lymph nodes dissection, in accordance with D1+b was performed by dissecting the lymph nodes around the celiac artery. The anastomosis of remaining stomach with layers, mucosa-submucosa and sero-muscular suture, was performed by hand for laparotomy, and the gastro-gastrostomy was performed by linear stapler in case of the laparoscopic surgery (Fig. 2, 3). The pyloroplasty was not performed and the nasogastric tube was not inserted. The oral intake started from the 4th day after the surgery.
Age, gender and body mass index (BMI), before surgery, were surveyed. The surgery safety was confirmed by the operation time, the length of free resection margin, tumor infiltration, dissected number of lymph nodes and early complication after the surgery, based on an independent T-test and the Fisher's exact test. To evaluate nutrition after surgery, BMI and albumin and hemoglobin level, at before and after the surgery, were analyzed by a T-test. The statistics were analyzed by the SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
The early complication is defined as within 30 days after the surgery or hospitalization period, and includes anastomotic leakage, bleeding, intestinal obstruction, respiratory complication, and reflux esophagitis. The late complication is defined as the reflux esophagitis 30 days after the surgery and anastomotic stricture. The gastric cancer stage was categorized based on the 7th edition of the American Joint Committee on Cancer (AJCC) and the histological features were defined based on the World Health Organization (WHO) classification and the Lauren classification.
Digestion-related symptoms include soring pain on the upper abdomen, burning feeling on the chest and dysphagia. In addition, gastroscopy was performed to check the recurrence of reflux esophagitis and anastomotic stricture. The reflux esophagitis was classified based on the Los Angeles (LA) classification.(7)
The mean age of 10 patients, which consisted of 5 male and 5 female, was 56.5±0.5 (40~75). Their mean hospitalization period after the surgery was 10.9±0.5 days (8~15 days) and the mean follow-up period was 30.8 months (12~64 months). No recurrence appeared. Nine of the patients had laparotomy and one patient (No. 9) had laparoscopic surgery. The mean operation time was 188.5±0.5 minutes (150~270 minutes) and the longest time was 270 minutes for the laparoscopic surgery. The T-stage consisted of 9 patients for T1 and 1 patient for T2, and all the patients had N0 of the N-stage. The mean number of retrieved lymph nodes was 25.2±0.5 (10~38). The mean length of free resection margin was 3.1±0.1 cm for the proximal parts and 3.7±0.1 cm for the distal parts (Table 1).
BMI, albumin and hemoglobin levels were measured to evaluate the nutritional state before and after the surgery. BMI significantly decreased from 23.8±3.4 kg/m2 before the surgery to 22.5±3.2 kg/m2 12 months after the surgery (P=0.021). The albumin level did not show significant changes from 4.8±0.2 g/dl before the surgery to 4.6±0.2 6 g/dl months after the surgery (P=0.851). The hemoglobin level showed significant changes from 13.8±1.5 g/dl before the surgery to 13.0±1.5 g/dl on 12 months after the surgery (P=0.016). However, one of the 10 patients (No. 10) was excluded because the patient had the surgery 9 months before and had no replacement data for BMI and hemoglobin level after 12 months.
Early complications, after the surgery, included 1 surgical site infection (No. 3) and 1 bleeding after the surgery (No. 5), and 2 patients (No. 6 and No. 8) sporadically showed dysphagia in 2 weeks after the surgery, but they showed improvement in 4 weeks after the surgery, without performing medical treatments. However, 1 patient (No. 4) showed reflux symptoms in 2 weeks after the surgery and medication with the proton pump inhibitor, along with antacid, was performed due to observing reflux esophagitis (LA classification C), despite no anastomotic stricture in the endoscopic result, and the medication was terminated due to improvements 2 months after the surgery. No other complications, including anastomotic leakage, were discovered. All other patients were discharged after a liquid diet (Table 1).
Only 1 patient, showing reflux esophagitis in the endoscopic exam before the surgery (No. 5), exhibited no symptoms after the surgery. Other 9 patients showed no particular findings, except early cancer lesions in the endoscopic results before the surgery, and did not have particular symptoms.
Three patients (Nos. 3, 6 and 8) complained of temporary dyspepsia after discharge. One patient (No. 3) suffered from soring pain a little bit on the upper abdomen and sporadic food reflux 1 month after the surgery, but the symptoms improved in the clinical tracing 2 months after without any specific treatment, including medication (Table 2).
All patients, except 1, who had treatment 2 months after the surgery due to gastroesophageal reflux symptom, performed endoscopic examinations on a regular basis 6 months after the surgery, and then on 1 year basis thereafter. The result showed that 1 (No. 4) out of 10 patients showed reflux esophagitis, but no anastomotic stricture. Gastric emptying scan was not taken routinely, but no patients showed symptoms related to the delay. Endoscope showed a wider anastomosis after gastro-gastrostomy (Fig. 4).
There are debates regarding the upper gastric cancer surgery. The excision range may be defined as the total gastrectomy or proximal partial gastrectomy, considering oncological or nutritional importance. The total gastrectomy shall be performed for the standard D2 excision in accordance with the principles of oncological surgeries. The 5th and the 6th lymph nodes are the 2nd lymph node group in the proximal gastric cancer, and it shall be dissected in the origin of the right gastroepiploic vessels and the right gastric vessels for complete separation, severing the blood circulation on the original part and the total gastrectomy is required.
The 2nd lymph nodes have low possibility of metastasis in the early gastric cancer, and the 5th and the 6th lymph nodes may not be completely separated through ligation of the right gastric artery and the right gastroepiploica vessels judging the D2 dissection, meaning that the proximal partial gastrectomy removing the gastric cancer lesions and their peripheries.
Generally, the total gastrectomy was performed to secure a wider lymph nodes dissection and a safer free resection margin as a surgical method of the upper gastric cancer, but has caused issues in the quality of lives due to improper nutrition and reflux among most patients.(5,6)
The proximal partial gastrectomy maintains food storage with remaining stomach and physiological functions, including the adjustment of hormones in the gastrointestinal tracts and mixing with digestive juice passing through the duodenum, mitigating symptoms, including anemia and malnutrition, following the total gastrectomy.(8-10) The study evaluated nutritional status by measuring the changes of BMI, albumin and hemoglobin before and after the surgery. BMI and hemoglobin level showed a significant decrease, but the albumin level did not show significant difference. BMI and hemoglobin level generally decreased, depending on dietary habits and food intake before and after the surgery, but its superiority may not be validated due to a failure of making a comparison to other reconstruction methods. However, the study introduced the CPPG and may become a comparison study on nutritional status with other reconstruction methods after verifying oncological safety.
The proximal partial gastrectomy, including the cardia, loses the sphincter function of lower esophagus and causes the gastroesophageal reflux and the reflux esophagitis. Severe reflux esophagitis may be developed to the secondary complication of the esophagus stricture and has a high possibility of the esophagogastric anastomotic stricture, due to a small lumen of the esophagus.
However, the CPPG conserves the cardia, unlike the conventional proximal partial gastrectomy, which totally excises the upper stomach and performs anastomosis of the esophagus to the remaining stomach, and excises lower than the E-G junction. It conserves the LES existing from the esophagus to the cardia to prevent gastroesophageal reflux and widens the anastomotic area through the anastomosis on the stomach, with wider internal cavity, with the purpose to prevent anastomotic stricture.
The most important defense mechanism of gastroesophageal reflux is LES and PEL. The abdominal esophagus with 4 cm size plays a role as the sphincter by pressure from the cavity and inhibits gastroesophageal reflux and PEL fixes LES. It is very crucial to prevent gastroesophageal reflux, considering that the reason of the type 1 hiatal hernia causing gastroesophageal reflux is an excessively loosened PEL, and E-G junction is placed inside the thoracic cavity, rather than on the abdomen cavity.
In proximal partial gastrectomy, the reflux esophagitis may be caused by losing gastroesophageal reflux defense mechanism by resecting the lower esophagus and performing the esophago-gastric anastomosis on the upper part of the E-G junction. The reflux esophagitis may be inhibited by preserving LES and PEL playing the most important role in preventing gastroesophgeal reflux after performing gastro-gastrostomy, and removing the proximal stomach after cutting the stomach, except 2 cm for anastomosis on the lower part of the E-G junction without hurting LES and PEL. However, the stomach shall be resected on the lower part of the E-G junction, and this operation shall be applied to the upper early gastric cancer where the cancer appears 4 cm, and further from the E-G junction considering the safe margin of the early gastric cancer.
In addition, the total gastrectomy completely dissected the 1st and the 2nd lymph nodes and detaches the lower esophagus, inevitable to damage the inferior phrenic artery and vein. This reduces the blood supply from the esophagus and causes anastomotic leakage or stricture. However, the CPPG additionally preserves the inferior phrenic artery and vein, and causes no damage to the blood supply.
The CPPG maintains nutritional advantages with the conventional proximal partial gastrectomy and prevents the gastroesophageal reflux and anastomotic stricture, compared to proximal gastrectomy, beneficial to upgrading the quality of lives than total gastrectomy.
However, No. 4 patient complained of reflux esophagitis symptom and had no structural problems in the endoscopic result after the surgery, and 30.0 kg/m2 of BMI, meaning that the patient is obese compared to other patients and estimating that the gastric emptying delay was accompanied due to frequently taking fats. It is considered to perform additional evaluation after forming more patient groups.
The study showed that the CPPG was technically feasible and safe for the upper early cancer, based on the incidence rates of complications and subsequent studies are required for oncological safety. However, it is a good formula for comparing other surgeries.
However, the CPPG is limited to performing the early cancer located below 4 cm, and further from the E-G junction to secure the free resection margin and the limitation includes that this study has only a small number of patients; 10 patients. The proximal upper excision shall preserve the right gastric vessels and the right gastroepiploic vessels, and the perfect D2 dissection may have failed, meaning that the method shall be limited to early gastric cancer with a rare possibility of the 5th and the 6th lymph nodes metastasis.
Recently, some results are reported that other restoration methods after the proximal partial gastrectomy may improve the reflux esophagitis and anastomotic stricture, as well as an increase in the quality of lives.(11,12) Sakuramoto et al.(13) reported that the crease method on the lower stomach with Touper type may improve the reflux and reflux esophagitis, and Jung et al.(14) reported that the dual path, using the proximal partial gastrectomy and residual gastric vestibular region lowered the degree of the reflux esophagitis.
In conclusion, the CPPG is technically feasible and is a safe procedure based on the results of the study; despite the indication that limits the performance. It is considered that further researches are required with large scaled prospected studies to establish the resection of upper early gastric cancer and restoration methods.
Figures and Tables
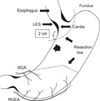 | Fig. 2Resection lines for cardia preserving proximal gastrectomy. RGA = right gastric artery; RGEA = right gastroepiploic artery; LES = low esophageal sphincter. |
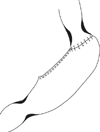 | Fig. 3Final configuration of anastomosis with suture lines after cardia preserving proximal gastrectomy. |
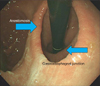 | Fig. 4Endoscopic follow-up of cardia preserving proximal gastrectomy showing wider anastomotic ring. |
References
1. Hyung WJ, Kim SS, Choi WH, Cheong JH, Choi SH, Kim CB, et al. Changes in treatment outcomes of gastric cancer surgery over 45 years at a single institution. Yonsei Med J. 2008. 49:409–415.

2. http://kostat.go.kr/portal/korea/index.action/. Accessed December 8, 2011.
3. An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008. 196:587–591.

4. Kim EM, Jeong HY, Lee ES, Moon HS, Sung JK, Kim SH, et al. Comparision between proximal gastrectomy and total gastrectomy in early gastric cancer. Korean J Gastroenterol. 2009. 54:212–219.

5. Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002. 26:1150–1154.

6. Olbe L, Lundell L. Intestinal function after total gastrectomy and possible consequences of gastric replacement. World J Surg. 1987. 11:713–719.

7. Sugiura T, Iwakiri K, Kotoyori M, Kobayashi M. Relationship between severity of reflux esophagitis according to the Los Angeles classification and esophageal motility. J Gastroenterol. 2001. 36:226–230.

8. Kim JH, Park SS, Kim J, Boo YJ, Kim SJ, Mok YJ, et al. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. 2006. 30:1870–1876.

9. Hinoshita E, Takahashi I, Onohara T, Nishizaki T, Matsusaka T, Wakasugi K, et al. The nutritional advantages of proximal gastrectomy for early gastric cancer. Hepatogastroenterology. 2001. 48:1513–1516.
10. Schwarz A, Büchler M, Usinger K, Rieger H, Glasbrenner B, Friess H, et al. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996. 20:60–66.

11. Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005. 29:1592–1599.

12. Matsushiro T, Hariu T, Nagashima H, Yamamoto K, Imaoka Y, Yamagata R, et al. Valvuloplasty plus fundoplasty to prevent esophageal regurgitation in esophagogastrostomy after proximal gastrectomy. Am J Surg. 1986. 152:314–319.

13. Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Moriya H, et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg. 2009. 209:344–351.

14. Jung HJ, Kim DH, Kim DH. Proximal gastrectomy with double tract reconstruction using the remnant antrum in early opper gastric cancer. J Korean Surg Soc. 2008. 74:261–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download