Abstract
Purpose
Mechanical stapler is regarded as a good alternative to the hand sewing technique, when used in gastric reconstruction. The circular stapling method has been widely applied to gastrectomy (open orlaparoscopic), for gastric cancer. We illustrated and compared the hand-sutured method to the circular stapling method, for Billroth-II, in patients who underwent laparoscopy assisted distal gastrectomy for gastric cancer.
Materials and Methods
Between April 2009 and May 2011, 60 patients who underwent laparoscopy assisted distal gastrectomy, with Billroth-II, were enrolled. Hand-sutured Billroth-II was performed in 40 patients (manual group) and circular stapler Billroth-II was performed in 20 patients (stapler group). Clinicopathological features and post-operative outcomes were evaluated and compared between the two groups.
In spite of improvement in the survival of patients with gastric cancer, it is still one of the most common causes of cancer related deaths in the world,(1,2) and gastric cancer surgery is one of the most common surgeries that have been performed for malignant gastrointestinal diseases. Since Billroth described his procedure of reconstruction in 1881, more than 100 years have passed. With the improved instrumentation used in gastric surgery and accumulation of surgeons' experiences, many technical skills and instruments have been developed for gastric surgery. These days, mechanical staplers are regarded as a good alternative to the hand sewing technique when used in gastric reconstruction. Moreover, laparoscopy assisted gastrectomy (LAG) has become a popular practice, and its application has increased significantly in the surgical management of gastric cancer in recent years. In LAG, mechanical staplers are very important instruments during surgery. Mechanical staplers have shortened the operation time, facilitated the performance of gastrointestinal anastomoses, and lowered the likelihood of suture failure, especially at sites where optimal gain of the surgical field was difficult.(3-5)
There have been many studies examining the use of staplers in Billroth-I anastomosis and the surgical technique of staplers.(6-11) However, there have been only a few studies reporting the use of circular staplers in Billroth-II anastomosis.(12-14) We compared the hand-sutured method to the circular stapling method for Billroth-II in patients who underwent laparoscopy assisted distal gastrectomy (LADG) for gastric cancer. In addition, we illustrate our procedures for the use of the circular stapler technique in Billroth-II anastomosis.
Between April 2009 and May 2011, 185 laparoscopy assisted gastrectomies for gastric cancer were performed by a single surgeon. If the tumor was located in the antrum of the stomach, we usually performed Billroth-I anastomosis. However, if the tumor was located in the lower body or mid-body of the stomach, we preferred Billroth-II anastomosis to preserve the safety of the proximal margin. Of 185 patients who had laparoscopy assisted gastrectomies, those who underwent the procedure during the surgeon's learning curve for LADG and patients who underwent total gastrectomy and distal gastrectomy with Billroth-I were excluded. Finally, the remaining 60 patients who underwent gastrectomy with Billroth-II were enrolled in this study. Hand-sutured Billroth-II was performed in 40 patients (manual group), and circular stapler Billroth-II was performed in 20 patients (stapler group). Several factors, including clinicopathological features (sex, age, body mass index, the presence of comorbidity, tumor size, tumor location, histologic type, length ofthe resection margin, T stage, N stage, and number of resected lymph nodes) and postoperative outcomes (operative time, the time for anastomosis, postoperative hospital stay, first flatus time, complication, symptoms related anastomosis) were evaluated and compared between the two groups. Gastric cancer stage was classified according to the 7th edition of the American Joint Committee on Cancer staging criteria.(15) All the values were expressed as means±standard deviations.
All patients were managed routinely using a standardized perioperative protocol that included the following: (1) if the tumor was located in the mid-body of the stomach, preoperative endoscopic clipping for localization was performed just proximal to the tumor, (2) no nasogastric intubation or preoperative mechanical bowel preparation was performed, (3) minimal spillage of gastric contents occurred, (4) one or two closed suction drains were used, (5) the patient had sips of water 48 hours after the operation, (6) a clear liquid diet was given 3 days after the operation, (7) the patient was discharged 6 or 7 days after surgery on a soft diet with no clinically abnormal symptoms.
As for postoperative anastomosis associated symptom, patients who complained of food stasis and had radiologic evidence of stasis of food material were defined as those with food stasis. Diet discomfort was defined when they didn't have radiologic evidence of food stasis but had subjective symptoms.
Chi-square and independent t-tests were used to compare the clinicopathologic factors and postoperative outcomes using GraphPadInStat® (version 3.06, GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was assumed for P-values<0.05.
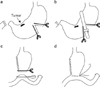
All laparoscopy-assisted distal gastrectomies were performed according to the standard procedure guidelines as described in a previous report.(16) For Billroth-II anastomosis, a 4~5 cm upper midline skin incision was made from the xiphoid area, and an incision template (Alexis™ Wound retractor, Applied Medical, Rancho Santa Margarita, CA, USA) was applied on the incision site. Hand-sutured technique of Billroth-II gastrojejunostomy was adopted as in conventional open surgery. After pulling out the stomach via the mini-laparotomy site, we applied a straight clamp on the side of the greater curvature for transection. After transecting the stomach, we applied linear stapler (Proximate linear cutter 100 mm, Ethicon Endo-Surgery, Cincinnati, OH, USA) for complete transection of the stomach. Hand-sutured Billroth-II gastrojejunostomy was then performed (Fig. 1). In the case of stapled Billroth-II gastrojejunostomy, after extracting the jejunum, we gently held up the jejunal wall, including the mucosa and a segment that was 20 cm distal to the ligament of Treitz. A purse-string clamp was applied at the jejunal wall. An anvil was inserted into the opening of the jejunum, and the purse-string was tightened. We then pulled out the stomach via mini-laparotomy, and the greater curvature side of the proximal resection margin was transected with a flexible laparoscopic stapling device (Echelon Flex 60 mm, Ethicon Endo-Surgery). After a small entry hole that was located opposite to the area of the tumor was opened in the distal stomach, which would be resected, the shaft of the circular stapler (CDH29, Ethicon Endo-Surgery) was introduced into the remnant stomach through this opening. The shaft of the circular stapler was rotated toward the side of greater curvature and the posterior wall of the remnant stomach, and the knob was twisted to extend the trocar in order to perforate the side of greater curvature and posterior wall of the remnant stomach. After the anvil was conjoined with the trocar, we fully tightened it, fired, and then maintained a squeeze of the handle for 15 seconds for hemostasis. Finally, we transected the distal stomach that was to be resected using the linear stapler to successfully complete the gastrojejunostomy (Fig. 2). For prevention of bleeding on the anastomosis line, we added interrupted sutures to the area.
The clinical and pathological characteristics of the 60 patients are summarized in Table 1. No significant differences were observed in clinicopathologic parameters between the two groups such asage, gender, body mass indexcomorbidity disease, tumor size, tumor location, histologic type, resection margin, Tstage, N stage, and number of retrieved lymph node. Operation times were significantly shorter in the stapler group (P=0.004). The times required for Billroth-II anastomosis were also significantly different between the groups (P<0.001). There were no statistically significant differences in postoperative hospital days, first flatus times, and general (early and late) complications. Median follow-up periods of manual group and stapler group were 15.1 and 5.1 months, respectively (P<0.001). Anastomosis related symptoms in the manual group occurred in 4 cases (food stasis in 1 case, diet discomfort in 3 cases). In the stapler group, there were 2 cases of symptoms related to anastomosis (diet discomfort in 2 cases). However, there was no statistically significant difference between the two groups (Table 2).
After the introduction of Billroth's procedure of gastrectomy and reconstruction, surgical techniques of gastric surgery have advanced gradually. Mechanical staplers have been used for gastrointestinal surgery since the latter half of 1970s.(17) Now, mechanical staplers have widely been used during open or laparoscopic gastrectomy for gastric surgery. Moreover, as laparoscopic surgery increases, mechanical staplers are very important instruments during operation. Initially, the circular stapler was only indicated for esophagojejunostomy, which was very difficult to perform with hand-suturing techniques.(18) Since then, there were many studies in the 1980s comparing mechanical stapling with hand suturing for esophagojejunostomy after total gastrectomy.(19-21) Many studies showed no significant difference in the occurrence of suture failure between mechanical stapling and hand suturing. Recently, improved instrumentation and increased experiences in handling have apparently led to a decrease in the incidence of suture failure.
In spite of reports of several techniques described for Billroth-I reconstruction with a mechanical stapled anastomosis,(6-11) there have been only a few studies using staplers in Billroth-II anastomosis.(12-14) A previous study compared Billroth-II anastomoses done by mechanical stapler to those done by hand suturing for reconstruction following distal gastrectomy.(12) Among 474 patients with hand-sutured anastomoses studied by Weil and Scherz,(12) 12 had suture failure, bleeding, or stenosis. In contrast, none of the 71 patients who had undergone mechanically stapled anastomoses had those complications. The study concluded that mechanical stapling was superior to hand suturing.
We usually performed Billroth-I anastomosis for reconstruction of distal gastrectomy, because it is simpler and more physiological than other types of reconstruction. When Billroth-II anastomosis for reconstruction was needed, we have thus far performed the anastomosis using the hand-sutured or linear stapler technique. Recently, we tried to perform Billroth-II anastomosis using the circular stapler. Regarding the postoperative outcomes in our series in both groups, we performed the anastomosis more quickly using the circular stapler than the hand-sutured method. Thus, total operation times of LADG were shorter using the circular stapler than using the hand-sutured method.
Our reconstruction technique for gastrojejunostomy has several merits over the hand-sutured method. First, we performed the anastomosis more quickly than when using the hand-sutured method. Thus, total operation times of LADG using the circular stapler are shorter than those of using the hand-sutured method. Second, since we make an anastomosis at the greater curvature of the remnant stomach, we can minimize the tension of B-II anastomosis and secure a sufficient resection margin. Third, because the circular stapler is inserted through the opening in the resected stomach, no additional gastrostomy on the remnant stomach is-needed, and no additional gastric closure is required. Fourth, because the linear and circular stapler lines do not across, it will prevent ischemia of the vascular supply of the stapler line. Therefore, although there were a small number of cases of the circular stapler method of Billroth-II, we did not have anastomosis leakage or ischemia.
Although median follow-up periods are short and different between the two groups, there were 4 cases of early and late complications in the manual group and 2 in the staple group during each early follow-up period, but there was with no statistically significant difference.
Anastomosis related symptoms developed in 4 cases of the manual group, and in 2 of the stapler group. The two cases of the stapler group complained of dyspepsia and dietary discomfort but showed no evidence of food stasis on radiologic studies. Symptoms improved after conservative treatment. Both groups have so far shown no other remarkable symptoms that are associated with the anastomosis.
The drawbacks of this study include the retrospective design of a small number of cases and the possibility of bias in data. In fact, the authors cannot establish the clear-cut indication for the choice of anastomotic methods. As seen in median follow-up periods, circular staple method was advocated in the later period, the bias may accrue to the comparison between both groups. And the additional application of the staplers may cause increase in surgical fee which needs to be taken into account between two groups. Therefore, a prospective, randomized, controlled trial with available indications is essential to overcome those drawbacks. However, this type of anastomosis may be considered feasible, considering that its results were as good as those of hand sutured methods.
Our study comparing hand-sutured with circular stapled anastomosis demonstrates that using circular staples took a significantly shorter time to complete the anastomosis. However, this is not a prospective randomized study, and the number of cases was small. Furthermore, prospective comparative studies for gastrojejunostomy methods, such as the hand-sutured, circular stapler, and linear stapler techniques will be needed.
Figures and Tables
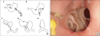
Fig. 2
Simplified illustration (A) and post-operative endoscopic finding (B) of the circular stapling anastomosis.
References
1. Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006. 24:2188–2196.

2. Hyung WJ, Kim SS, Choi WH, Cheong JH, Choi SH, Kim CB, et al. Changes in treatment outcomes of gastric cancer surgery over 45 years at a single institution. Yonsei Med J. 2008. 49:409–415.

3. Everett WG, Friend PJ, Forty J. Comparison of stapling and hand-suture for left-sided large bowel anastomosis. Br J Surg. 1986. 73:345–348.

4. Viste A, Haùgstvedt T, Eide GE, Søreide O. Postoperative complications and mortality after surgery for gastric cancer. Ann Surg. 1988. 207:7–13.

5. Kataoka M, Masaoka A, Hayashi S, Honda H, Hotta T, Niwa T, et al. Problems associated with the EEA stapling technique for esophagojejunostomy after total gastrectomy. Ann Surg. 1989. 209:99–104.

6. Arnaud JP, Ollier JC, Adloff M. A new procedure for Billroth-I anastomoses with the EEA stapler. Int Surg. 1983. 68:63–64.
7. Nakane Y, Kanbara T, Michiura T, Inoue K, Iiyama H, Nakai K, et al. Billroth I gastrectomy using a circular stapler to treat gastric cancer. Surg Today. 2001. 31:90–92.

8. Hori S, Ochiai T, Gunji Y, Hayashi H, Suzuki T. A prospective randomized trial of hand-sutured versus mechanically stapled anastomoses for gastroduodenostomy after distal gastrectomy. Gastric Cancer. 2004. 7:24–30.

9. Yang HK, Lee HJ, Ahn HS, Yoo MW, Lee IK, Lee KU. Safety of modified double-stapling end-to-end gastroduodenostomy in distal subtotal gastrectomy. J Surg Oncol. 2007. 96:624–629.

10. An JY, Yoon SH, Pak KH, Heo GU, Oh SJ, Hyung WJ, et al. A novel modification of double stapling technique in Billroth I anastomosis. J Surg Oncol. 2009. 100:518–519.

11. Kim T, Yu W, Chung H. Handsewn versus stapled gastroduodenostomy in patients with gastric cancer: long-term follow-up of a randomized clinical trial. World J Surg. 2011. 35:1026–1029.

12. Weil PH, Scherz H. Comparison of stapled and hand-sutured gastrectomies. Arch Surg. 1981. 116:14–16.

13. el Ferzli G, Worth MH Jr. Direct anastomotic visualization in stapled Billroth II gastrectomy. Surg Gynecol Obstet. 1986. 163:487–488.
14. Oh SJ, Hong JJ, Oh CA, Kim DH, Bae YS, Choi SH, et al. Stapling technique for performing Billroth II anastomosis after distal gastrectomy. J Gastrointest Surg. 2011. 15:1244–1246.

15. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer.
16. Kim MC, Kim KH, Kim HH, Jung GJ. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. 2005. 91:90–94.

17. Ravitch MM, Steichen FM. A stapling instrument for end-to-end inverting anastomoses in the gastrointestinal tract. Ann Surg. 1979. 189:791–797.

18. Nance FC. New techniques of gastrointestinal anastomoses with the EEA stapler. Ann Surg. 1979. 189:587–600.

19. Walther BS, Oscarson JE, Graffner HO, Vallgren S, Evander A. Esophagojejunostomy with the EEA stapler. Surgery. 1986. 99:598–603.
20. Paolini A, Tosato F, Cassese M, De Marchi C, Grande M, Paoletti P, et al. Total gastrectomy in the treatment of adenocarcinoma of the cardia. Review of the results in 73 resected patients. Am J Surg. 1986. 151:238–243.
21. Habu H, Kando F, Saito N, Sato Y, Takeshita K, Sunagawa M, et al. Experience with the EEA stapler for esophagojejunostomy. Int Surg. 1989. 74:73–76.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download