Abstract
Borrmann type 4 gastric cancers are notorious for the difficulty of finding cancer cells in the biopsy samples obtained from gastrofiberscopy. It is important to obtain the biopsy results for making surgical decisions. In cases with Borrmann type 4 gastric cancer, even though the radiological findings (such as an upper gastrointestinal series, abdominal computed tomography and positron emission tomography/computed tomography) or the macroscopic findings of a gastrofiberscopy examination imply a high suspicion of cancer, there can be difficulty in getting the definite pathologic results despite multiple biopsies. In these cases, we have performed endoscopic mucosal resection under gastrofiberscopy as an alternative to simple biopsies. Here we report on a case in which no cancer cells were found even in the endoscopic mucosal resection specimen, but the radiologic evidence and clinical findings were highly suspicious for gastric cancer. The patient finally underwent total gastrectomy with lymph node resection, and she was pathologically diagnosed as having stage IV gastric cancer postoperatively.
Advanced gastric cancer is classified as the Borrmann types based on the characteristic macroscopic morphology. Among them, Borrmann type 4 gastric cancer is referred to as linitis plastica or scirrhous carcinoma, which has the characteristic of diffuse infiltration that occupies a large area of the stomach, serous infiltration and frequent lymph node metastasis.(1)
The primary lesions of Borrmann type 4 are different from other macroscopic types of gastric cancer as the former infiltrates the submucosal layer and the disease progress and specific findings in the mucosa layer are not sufficient for making a diagnosis, and so making an early diagnosis is difficult. In addition, peritoneal metastasis is common at the time of its detection, and so therapeutic resection is difficult and the prognosis is very poor.(2,3)
Endoscopic gastric mucosal resection performed prior to surgery for making the diagnosis is a procedure that comprehensively examines the lesions endoscopically. It lifts the lesion by the submucosal injection of a mixture of physiological saline, epinephrine and indigo carmine, and the mucosa is then resected. This procedure has recently been widely applied for the treatment of early gastric cancer. At our hospital, for a case that cancer cells could not be detected by repeated endoscopic biopsy, we performed histological tests on the endoscopically resected gastric mucosa, but cancer cells still could not be detected. Based on the radiological test findings, the endoscopic macroscopic characteristics and the characteristics of the clinical course, we performed total gastrectomy and lymphadenectomy. The tissues obtained from the surgery were examined, and the patient's disease was determined to be Borrmann's type 4 gastric cancer.
A 45 years old female was admitted for nausea, vomiting and anorexia, and this had all started 6 months previously. For her past history, she was diagnosed with hypertension 10 years ago, she was diagnosed as having IgA nephropathy 5 years ago and she was taking beta-blocker, Cozaar and angiotensin converting enzyme inhibitors. There was no significant family history. The vital signs were normal at the time of admission. On the abdominal physical examination, a hard and mobile mass the size of a baby's fist was palpated in the upper abdomen and this was associated with pain. Any other special findings were not detected on the physical examination. The results of the general blood tests that included tumor markers were all normal.
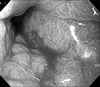
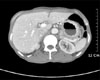
On gastroduodenoscopy, the mucosa of the body of the stomach was not well spread, and the gastric folds were thicker and harder than normal, and so we suspected this to be Borrmann's type 4 stomach adenocarcinoma that showed hypertrophic gastric lesions, and then a biopsy was performed (Fig. 1). On the abdominal computed tomography, similarly, the layering of the stomach wall was lost and the pattern that the stomach was not well spread suggested linitis plastica corresponding to Borrmann's type 4 stomach adenocarcinoma (Fig. 2).
On gastroduodenoscopy and ultrasonography, the greater curvature of the stomach wall showed diffuse thickening, and the thickness was observed to be approximately 10 mm. Particularly, the 2nd layer was thickened and heterogeneous low echo contrast was primarily seen, and these findings corresponded to hypertrophic gastric disease or type 4 advanced stomach cancer. When performing a gastroduodenal barium enema, we observed that the entire stomach wall was thickened and the stomach space was narrowed. In addition, there was the loss of peristaltic movement.
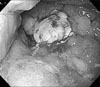
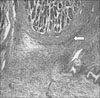
Gastric cancer was suspected and so endoscopic biopsy performed, but cancer cells were not detected. Nonetheless, several imaging findings and the clinical symptoms strongly suggested stomach cancer. Thus, endoscopic mucosal resection of the stomach wall was performed for making the diagnosis (Fig. 3). After the mucosal resection of the hypertrophic lesions of the greater curvature and the anterior wall of the body, tissues were taken by biopsy forceps from the area determined to be the submucosal layer and histological tests were performed. Nevertheless, cancer cells were not detected on the histological tests, and only the thickening of epithelial cells was observed (Fig. 4).
Stomach cancer was strongly suspected and surgery was recommended. However, the patient refused to undergo surgery without a definite histological diagnosis. Ambulatory follow-ups were planned and the patient was discharged. During the follow-up observation, the clinical symptoms of the patient deteriorated, and surgery was again recommended. The patient agreed to undergo surgery and laparotomy was performed. During laparotomy, adhesion or ascites was not detected. In addition, there were no findings of metastasis to other organs within the abdominal cavity and the rectal shelf, and dissemination in the peritoneum was not observed. The stomach wall was thickened overall, and the local enlargement of lymph nodes in the vicinity of the stomach was observed, so total gastrectomy, extended lymphadenectomy and Roux-en-Y esophagojejunostomy were performed. The stomach disease was diagnosed as poorly differentiated type 4 advanced stomach cancer by the postsurgical pathohistological tests. The mucosa layer was thickened to 5~9 mm, and most of the cancer cells were found as a pattern that initiated from the submucosal layer and then it spread (Fig. 5). This was the diffuse type according to Lauren's classification. Tumor had penetrated and invaded the serous layer. Cancer cell metastasis was detected in 23 of the 35 resected lymph nodes and the final disease stage was determined to be stage IIIc (T4aN-3bMo) according to the AJCC TNM staging system (7th ed).
After surgery, the patient was discharged without any special complications. Combination chemotherapy with TS-1 and cisplatin was initiated from 4 weeks after surgery. However, anorexia and severe bone marrow suppression developed during the 4th cycle, and so currently, the chemotherapy has been interrupted and the patient is under ambulatory follow-up observation.
The diagnosis of Borrmann's type 4 stomach cancer, which is an advanced state of scirrhous carcinoma, is difficult, and so the diagnosis is often delayed due to false negative endoscopic and histological tests. These tests can be negative because the characteristic of cancer cells that are in the area below the mucosa appears to be normal, and the cancer cells along the submucosal plane have an unclear boundary and they widely infiltrate into the vicinity. Kohli et al.(4) have reported that the initial endoscopic findings of Borrmann's type 4 stomach cancer may interpreted as the IIc type or III+IIc type, and these types have the macroscopic morphology of early stomach cancer. So, for making the diagnosis of Borrmann's type 4 cancer, attention should be paid to the shallow depressions and slight stiffness of the stomach wall. It has been reported that abdominal computed tomography has 89% sensitivity for making the diagnosis of Borrmann's type 4 stomach cancer, but it is not effective for making an early diagnosis.(5)
Because early detection is difficult, tumor progression could readily induce disseminated metastasis in the peritoneum, and then the rate of lymphatic metastasis is high, and there are reports of peritoneal metastasis occurring even after therapeutic resection. Cytochemical or molecular studies on such a particular growth and progression pattern are now ongoing.(6,7)
Because of the biological characteristic of Borrmann's type 4 stomach cancer that it tends to spread to the peritoneum, peritoneal metastasis is frequently detected at the time of diagnosis, and its prognosis is known to be poor. Nonetheless, clinicopathological factors that are significantly different from other types are associated with the disease stages in most cases, and these factors have been revealed to be closely associated with a delayed diagnosis. Because of a delayed diagnosis, already highly advanced disease stages are seen at the time of surgery and Borrmann's type 4 stomach cancer occupies the entire stomach in many cases and it can not be resected, and so non-curiative resection is frequently performed. Therefore, it is thought that presurgical chemotherapy may be one possible method to obtain a good prognosis by lowering the disease stage and increasing the resection rate,(8) and prospective randomized studies on such methods are now ongoing. Postsurgical complications develop at a significantly higher rate in patients with Borrmann type 4 disease as compared with that of the other macroscopic types of stomach cancer. This is thought to be due to that the frequency of combined resection of organs in the vicinity of stomach is high, the resection margin is positive for residual cancer in many cases and non-curative resection is performed in many cases.(1)
It has been reported that the characteristics of Borrmann's type 4 stomach cancer are the high frequency of undifferentiated carcinoma and the proliferation of interstitial cells.(1,9-11) Ichiyoshi et al.(12) have reported that such cancer cells primarily spread one dimensionally on the stomach wall and serous infiltration was overlooked in 40% of the cases when it was diagnosed macroscopically only during surgery, and then the opportunities to prevent peritoneal recurrence by intraperitoneal chemotherapy during surgery were lost. Presently, the new oral derivatives of 5-fluorouracil TS-1 have shown higher than anticipated treatment effectiveness in clinical studies.(13)
There is a high rate of a false negative diagnosis of Borrmann's type 4 stomach cancer by performing abdominal computed tomography and other imaging methods, as well as by an endoscopic diagnosis. Therefore, it is thought that endoscopic gastric mucosal resection, as a new treatment method for early stomach cancer, may be of help to diagnosis Borrmann's type 4 stomach cancer that is not clear on abdominal computed tomography or positron emission tomography/computed tomography or to diagnose local Borrmann's type 4 stomach cancer. However, as was noted in our case, in stomach tissues with highly hypertrophic mucosa, if the spread of cancer cells is initiated from the submucosal layer, then obtaining a biopsy that includes the submucosal layer may be very difficult when performing endoscopic stomach mucosal resection. The thickness of the stomach mucosal layer of our case was 5~9 mm, which was 2 times thicker than the normal thickness (3~4 mm). Therefore, if Borrmann's type 4 stomach cancer is strongly suspected prior to surgery and surgery is being considered, then deep resection of the stomach wall for obtaining the submucosal layer when perfoming endoscopic stomach mucosal resection may increase the diagnosis rate despite of the development of stomach perforation and complications.
Our patient was suspected to have Borrmann's type 4 stomach cancer, the cancer cells could not be detected even on the biopsy by endoscopic stomach mucosal resection and so it was a difficult case. We were able to histologically diagnose the cancer after total gastrectomy, and lymphadenectomy performed because of the deterioration of the clinical course.
It is well known that making the definite presurgical histological diagnosis of Borrmann's type 4 stomach cancer is difficult in many cases due to the characteristic development process of the tumor cells. For such cases, repeated endoscopic biopsy or deep biopsy that reaches the deep area of the stomach wall is required. For cases that cancer cells could not be detected despite of repeated biopsy, by considering the results of abdominal computed tomography, the upper gastrointestinal series and the clinical symptoms together, laparotomy can be decided on for many cases. In our case, cancer cells could not be detected despite perfoming 3 gastroscopic biopsies. Nonetheless, the findings of abdominal computed tomography, the upper gastrointestinal series and the clinical symptoms strongly suggested stomach cancer, and laparotomy was recommended to the patient. Yet the patient refused to undergo surgery without a definite histological diagnosis, so we performed stomach mucosal resection as an additional diagnostic method. Cancer cells could not be detected even with this procedure. However, the patient's clinical symptoms deteriorated and then the patient consented to undergo surgery. However, the cost of endoscopic stomach wall mucosal resection is high, and it has problems of complications associated with the procedure (hemorrhage, perforation of stomach wall, etc.). Hence, it may be attempted for very specially selected cases of suspected Borrmann's type 4 stomach cancer, for example, for suspected cases of local Borrmann's type 4 stomach cancer, or for cases whose results of abdominal computed tomography or positron emission tomography/computed tomography are not clear. Biopsy by gastroscopic ultrasonography(14,15) and endoscopic submucosal dissection may be additional presurgical diagnosis methods.
Figures and Tables
References
1. Kwon SJ, Lee GJ. Clinicopathologic characteristics of Borrmann type 4 gastric cancer. J Korean Surg Soc. 2003. 64:127–133.
2. Yook JH, Oh ST, Kim BS. Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer Res Treat. 2005. 37:87–91.

3. Ahn JS, Ryu SW, Kim IH, Sohn SS. Clinicopathological analysis of recurrent gastric cancer aft er curative resection. J Korean Surg Soc. 2003. 65:210–216.
4. Kohli Y, Takeda S, Kawai K. Earlier diagnosis of gastric infiltrating carcinoma (scirrhous cancer). J Clin Gastroenterol. 1981. 3:17–20.

5. Balthazar EJ, Siegel SE, Megibow AJ, Scholes J, Gordon R. CT in patients with scirrhous carcinoma of the GI tract: imaging findings and value for tumor detection and staging. AJR Am J Roentgenol. 1995. 165:839–845.

6. Yashiro M, Chung YS, Sowa M. Role of orthotopic fibroblasts in the development of scirrhous gastric carcinoma. Jpn J Cancer Res. 1994. 85:883–886.

7. Yoshida K, Kyo E, Tsujino T, Sano T, Niimoto M, Tahara E. Expression of epidermal growth factor, transforming growth factor-alpha and their receptor genes in human gastric carcinoma; implication for autocrine growth. Jpn J Cancer Res. 1990. 81:43–51.

8. Takahashi S, Kinoshita T, Konishi M, Nakagouri T, Inoue K, Ono M, et al. Phase II study of sequential high-dose methotrexate and fluorouracil combined with doxorubicin as a neoadjuvant chemotherapy for scirrhous gastric cancer. Gastric Cancer. 2001. 4:192–197.

9. Kim DY, Kim HR, Kim YJ, Kim SK. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002. 72:739–742.

10. Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, et al. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995. 58:112–117.

11. Hamy A, Letessier E, Bizouarn P, Paineau J, Aillet G, Mirallié E, et al. Study of survival and prognostic factors in patients undergoing resection for gastric linitis plastica: a review of 86 cases. Int Surg. 1999. 84:337–343.
12. Ichiyoshi Y, Maehara Y, Tomisaki S, Oiwa H, Sakaguchi Y, Ohno S, et al. Macroscopic intraoperative diagnosis of serosal invasion and clinical outcome of gastric cancer: risk of underestimation. J Surg Oncol. 1995. 59:255–260.

13. Kinoshita T, Konishi M, Nakagohri T, Inoue K, Oda T, Takahashi S, et al. Neoadjuvant chemotherapy with S-1 for scirrhous gastric cancer: a pilot study. Gastric Cancer. 2003. 6:Suppl 1. 40–44.

14. Green J, Katz S, Phillips G, Bank S, Ilardi C, Hadju E, et al. Percutaneous sonographic needle aspiration biopsy of endoscopically negative gastric carcinoma. Am J Gastroenterol. 1988. 83:1150–1153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download