Abstract
The natural course of untreated patients with signet ring cell carcinoma of the stomach remains poorly understood while assumptions have been made to distinguish it from other types of gastric cancer. A 74-year-old Korean woman was diagnosed with early gastric cancer with signet ring cell histology and refused surgery. A satellite lesion was identified 46 months after the initial diagnosis. The patient finally agreed to undergo distal subtotal gastrectomy 53 months following the initial diagnosis. Postoperative histological examination of both lesions confirmed signet ring cell carcinoma associated with submucosal invasion. There was no evidence of lymph node metastasis.
There are a number of limitations in studying the natural course of gastric cancer. Most patients undergo surgery with or without adjuvant chemotherapy once the diagnosis has been established. Patients who do not receive treatment tend to have developed very advanced disease. To undertake a study on the natural course of gastric cancer, the patient population is confined to those who have refused to undergo treatment. The clinicopathological characteristics of signet ring cell carcinoma of the stomach are known to differ from that of other types of gastric cancer.(1) Some have reported a higher rate of forming multiple gastric cancers if the primary lesion is signet ring cell carcinoma.(2) Thus far, there is a lack of information on the natural course of signet ring cell carcinoma of the stomach.
We herein report a rare case of signet ring cell carcinoma of the stomach in a female patient who declined early surgical intervention, whose disease developed a satellite lesion that did not progress to advanced gastric cancer 53 months after the initial diagnosis.
A 74-year-old healthy Korean woman presented with a history of intermittent epigastric pain. Her husband and son were known to have developed gastric cancer. The patient underwent endoscopic examination of the stomach in December 2005, which demonstrated a depressed mucosal lesion in the anterior wall of the distal gastric body (Fig. 1). Histological examination revealed signet ring cell carcinoma. Abdominal computed tomography (CT) excluded gastric wall thickening, lymphadenopathy and distant metastasis. The diagnosis was type IIb early gastric cancer for which surgery was recommended, but she declined for an unspecified reason.
The patient took antacids and digestive medicines irregularly, and underwent a repeat endoscopy in September 2006, which showed no interval changes. However, at the third endoscopy in October 2009, a satellite lesion was detected in the greater curvature of the distal gastric body adjacent to the previously confirmed malignant lesion. Histological examination of the satellite lesion confirmed signet ring cell carcinoma of the stomach, identical to the primary lesion. A CT scan showed no interval changes and the patient continued to decline surgery. In March, 2010, while no interval changes were shown on repeat endoscopy, she finally agreed to undergo surgery.
The pre-operative physical examination was unremarkable. The levels of carcinoembryonic antigen and carbohydrate antigen 19-9 were normal. All routine laboratory test results were within normal limits.
She underwent open curative distal subtotal gastrectomy, stapled Billroth I gastroduodenostomy and D2 extended lymphadenectomy in May 2010. Two type IIb early gastric cancer lesions were located in the anterior wall and greater curvature of the distal gastric body without associated metastatic lesions in the resected lymph nodes (Fig. 2).
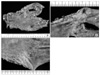
Postoperative histological examination confirmed invasion of signet ring cell carcinoma into the gastric submucosal layer (Fig. 3). The primary lesion measured 3.0×4.5 cm on the anterior wall of the distal gastric body, and the satellite lesion measured 2.0× 2.5 cm on the greater curvature of the distal gastric body. The two lesions were pathologically not connected. There was no evidence of metastasis in the 40 lymph nodes resected. The final diagnosis was stage Ia signet ring cell carcinoma of the stomach, based on the seventh edition of Union for International Cancer Control classification.
There have been efforts to define the natural course of gastric cancer. However, the disease course in most patients studied were altered by chemoradiation or endoscopic intervention.(3,4) Very few studies have described the natural course of gastric cancer by studying patients who have refused to undergo treatment, or whose surgical resection was delayed. The median period for early gastric cancer to progress into advanced gastric cancer then death are 37~44 months and 77 months, respectively.(5,6) A study on patients with early gastric cancer of the cardia reported an average survival time of 53.3 months.(7)
A report exists in the literature on a patient with early gastric cancer who underwent surgery 95 months after the initial diagnosis (Table 1).(8) Postoperative histological examination confirmed advanced poorly differentiated adenocarcinoma of the stomach. There is another report on a patient with advanced gastric cancer who underwent curative surgery 78 months after the initial diagnosis.(9) These reports are significant because they describe the outcomes of gastric cancer patients who underwent a significantly delayed curative surgery. However, it was impossible to determine whether the period for early gastric cancer to progress into advanced gastric cancer was extraordinarily long in these cases. Our patient underwent curative gastrectomy 53 months after the initial diagnosis. Her disease did not progress into advanced gastric cancer, suggestive of slower progression than cases reported previously.(5-7)
One of the reasons for such slow progression could be due to improved diagnostic tools that led to earlier diagnosis of gastric cancer.(10) However, there appears to be more than just diagnostic advancement in our case, which showed extraordinarily slow progression. Early gastric cancer with signet ring cell histology tends to be superficial and large, leading to earlier diagnosis than gastric cancer of other histological types.(1) The superficial spreading nature may be helpful for early detection, because it has less chance of being missed during endoscopy.
In addition to a superficial spreading nature, the formation of satellite lesions may be a significant characteristic of signet ring cell carcinoma. Despite the advancement in diagnostic tools, a proportion of gastric cancers often remain undetected.(11,12) Multi-focal gastric cancer is often missed, because they tend to be small and flat in appearance.(13) Our patient developed a satellite lesion 46 months after the initial diagnosis. On review of the images taken in December 2005, no suspicious lesion was identified at the site of the satellite lesion. The next images taken in October 2006 showed minimally elevated mucosa at the site of the eventual satellite lesion. However, at the time of endoscopy in October 2006, malignant change was not suspected at the site. We were unable to investigate endoscopic images between 2006 and 2009 because our patient had refused endoscopic examination during that period. Some reports assert that early signet ring cell carcinoma of the stomach tends to spread in the mucosal and submucosal layers continuously or discontinuously.(1,2) This may explain the prevalence of multiple gastric cancers in the signet ring cell carcinoma.
Some reports discuss the "collision tumor" phenomenon, in which multiple early gastric cancers fuse together to form an advanced gastric cancer.(14) In our case, it was impossible to exclude the possibility of two distinct early gastric cancers fusing together to form a single advanced gastric cancer. As an early gastric cancer, signet ring cell carcinoma is known to have a tendency to form multiple lesions. However, once the disease evolves into advanced gastric cancer, the diffusely infiltrating characteristics of signet ring cell carcinoma may be associated with a poor prognosis by involving the entire stomach, resulting in what is known as linitis plastica. (1,2)
Advancement in diagnostic tools along with signet ring cell carcinoma's tendency to spread superficially may have resulted in an apparently slow progression of this cancer. Although the results are not consistent, some reports discuss that, as an early gastric cancer, signet ring cell carcinoma has a favorable prognosis with less lymph node metastasis.(15) One may anticipate a consequent extension of endoscopic resection criteria for early signet ring cell carcinoma of the stomach. However, it must be emphasized that forming satellite lesions is another characteristic of signet ring cell carcinoma, for which extending endoscopic resection criteria is inappropriate.
Some may argue that interpreting an accumulation of cases of extraordinarily slow progression may lead to bias in understanding the natural course of gastric cancer. It is true that our case exhibited extraordinarily slow progression as far as our knowledge on the natural course of signet ring cell carcinoma of the stomach tells. However, it is inevitably true that our knowledge doesn't tell much about the natural course of signet ring cell carcinoma in specific. It would be interesting to investigate the natural course of signet ring cell carcinoma of the stomach not only for cases with unusual progression but also for cases with usual progression. By doing so, clinicians may be able to establish the natural course of signet ring cell carcinoma of the stomach, particularly concerning the time periods required 1) to infiltrate laterally and form satellite lesions, 2) to infiltrate in depth and become an advanced gastric cancer, and 3) to spread lymphatically and form a nodal metastasis. Special attention must be paid to the detecting of satellite lesions during endoscopy, particularly in patients with signet ring cell carcinoma.
Figures and Tables
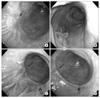 | Fig. 1Endoscopic images of the main lesion (single arrow) and the satellite lesion (double arrows) of early gastric cancer. The images were taken December 2005, when the initial diagnosis was made (A), subsequently on October 2006, 10 months after the diagnosis (B), on October 2009, 46 months after the diagnosis (C), and on March 2010, 51 months after the diagnosis (D). |
 | Fig. 2The resected specimen in May 2010. The main lesion (single arrow) of type IIb early gastric cancer, 3.0×4.5 cm in size, was located in the anterior wall of the distal body of the stomach. The satellite lesion (double arrows) of a type IIb early gastric cancer, 2.0×2.5 cm in size, was located in the greater curvature of the distal body of the stomach. The two lesions were separated by normal mucosa. (A) Specimen. (B) Main lesion. (C) Satellite lesion. |
 | Fig. 3Histopathologic findings. (A) The histopathologic findings of the main lesion were consistent with submucosal infiltration of tumor cells (H&E, ×200). (B) The tumor cells from the main lesion showed signet ring cell components (×400). (C) The histopathologic findings of the satellite lesion also showed submucosal infiltration of tumor cells (×200). (D) The tumor cells from the satellite lesion showed signet ring cell components as well (×400). |
Table 1
Characteristics from previously published case reports of patients with significantly delayed curative surgery for gastric cancer(8,9)

Stage defined by 7th edition of Union for International Cancer Control (UICC) classification. Nx denotes N staging without specified descriptions. EGC = early gastric cancer; AGE = advanced gastric cancer; Bor = Borrmann type; MOD = moderately differentiated adenocarcinoma; POOR = poorly differentiated adenocarcinoma. *Time interval between initial diagnosis and surgery; †Suspected peritoneal seeding on computed tomography.
Acknowledgments
This article was presented at The 30th Annual Conference of The Korean Gastric Cancer Association as a poster under the title of "A case of early gastric cancer forming a satellite lesion before being resected 53 months after the initial diagnosis".
References
1. Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992. 69:1645–1650.

2. Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK. Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion. 2010. 81:35–42.

3. Reed VK, Krishnan S, Mansfield PF, Bhosale PR, Kim M, Das P, et al. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008. 71:741–747.

4. Graham DY, Uemura N. Natural history of gastric cancer after Helicobacter pylori eradication in Japan: after endoscopic resection, after treatment of the general population, and naturally. Helicobacter. 2006. 11:139–143.

5. Tsukuma H, Mishima T, Oshima A. Prospective study of "early" gastric cancer. Int J Cancer. 1983. 31:421–426.

6. Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000. 47:618–621.

7. Guanrei Y, Songliang Q, He H, Guizen F. Natural history of early esophageal squamous carcinoma and early adenocarcinoma of the gastric cardia in the People's Republic of China. Endoscopy. 1988. 20:95–98.

8. Furukawa K, Yamada K, Konomi K, Tanaka M. Gastric carcinoma resected 95 months after being diagnosed: report of a case. Surg Today. 1994. 24:756–758.

9. Son HJ, Yoo MW, Kong SH, Ahn HS, Lee IK, Kim WH, et al. advanced gastric cancer that was curatively resected 78 months after being diagnosed: report of a case. J Korean Gastric Cancer Assoc. 2010. 10:40–44.

10. Yao K, Iwashita A, Tanabe H, Nagahama T, Matsui T, Ueki T, et al. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007. 5:869–878.

11. Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004. 36:874–879.

12. Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005. 17:1345–1349.

13. Oohara T, Aono G, Ukawa S, Takezoe K, Johjima Y, Kurosaka H, et al. Clinical diagnosis of minute gastric cancer less than 5 mm in diameter. Cancer. 1984. 53:162–165.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download