Abstract
Letrozole is a drug used in the treatment of postmenopausal women with breast and ovarian tumours. There is no evidence in the literature indicating its use in treating gastric cancer. We present a 68 year old lady admitted from the emergency department with weight loss, malaise and anaemia. Investigations confirmed the presence of two different primary tumours in the left breast and the stomach. Following that this patient with oestrogen receptor positive breast cancer and oestrogen receptor negative gastric cancer was treated with letrozole for her breast cancer followed by a gastric resection. Independent histology by two pathologists pre-operatively diagnosed gastric adenocarcinoma. Post-operatively, independent analysis of the resected stomach, omentum and lymph nodes revealed no evidence of gastric cancer. Therefore we conclude that there is a possibility of letrozole having an effect on gastric cancer. Further studies are needed.
Aromatase inhibitors (AI) are a class of drugs used in the treatment of breast and ovarian cancer in postmenopausal women. Estrogen has a great role on the progression of breast cancer, and studies have shown that exposure to estrogens increases the risk of breast cancer(1) therefore therapeutic agents targeting estrogen synthesis & Estrogen receptors have made a huge difference to the treatment of breast cancer. Letrozole is a very potent and selective AI that leads to the inhibition of the enzyme activity of intracellular aromatase at the major sites where it is found.(2) Aromatase is expressed primarily in the ovary and also in central and peripheral tissues, fat, muscle, liver, and breast. Letrozole is only effective in postmenopausal women in whom estrogen is produced predominantly by the adrenals. Whilst in premenopausal women estrogen is produced in the ovaries and letrozole is ineffective. Randomized clinical trials have demonstrated the clinical benefits of letrozole across the spectrum of hormone-responsive breast cancer in postmenopausal women.(3,4)
It may be very difficulty to differentiate between primary gastric tumours from metastatic breast cancer to the stomach on clinical, endoscopic, radiological and histopathological features(5,6) detailed immunohistochemical analysis may be the only consistent method for differentiating between metastatic and primary gastric carcinoma. Although oestrogen and progesterone receptor positivity in the gastric biopsies suggest breast cancer metastasis to the stomach, it is worth noting that oestrogen and progesterone receptor positivity have been reported in 32% and 12% of patients with primary gastric cancer.(7) However, these findings are based upon studies using first-generation antibodies against Oestrogen receptor β (ERβ) which are no longer used in standard practice. Taal et al investigated whether immunohistochemical detection with second-generation antibodies against Oestrogen receptor α (ERα) can be used to diagnose gastric metastasis of breast carcinoma(8) and they concluded that ERα expression can be reliably used to diagnose gastric metastasis of breast cancer. They also investigated if the expression pattern of E-cadherin could help in the differentiating the diagnosis of primary gastric cancer versus metastatic breast carcinoma. In their study absence of E-cadherin staining was significantly related to metastatic breast carcinoma. Therefore absence of E-cadherin expression in an adenocarcinoma in a gastric biopsy should raise the possibility of metastatic breast carcinoma and ERα positivity can be reliably used to diagnose gastric metastasis of breast carcinoma.
Positive monoclonal staining with gross cystic disease fluid protein-15 has been found to be a sensitive (55~76%) and specific (95~100%) marker to correctly identify a malignant lesion as metastatic breast carcinoma.(9-12)
Table 1 summarizes the Immunohistochemical methods of Differentiating Primary gastric cancer from Metastatic gastric cancer.
To summarize, in patients with a history of breast cancer, one should suspect the possibility of breast cancer metastasis to the stomach when new primary gastric cancer is diagnosed. Complete histopathological and immunohistochemical analysis of the gastric biopsies and comparison with the original breast cancer pathology is important.(13)
We present a 68 year old lady who was admitted as an emergency with malaise, weight loss & haemoglobin of 3 g/dl. She had not presented previously as she had been caring for her terminally ill husband. She underwent a gastroscopy to investigate her anaemia which revealed a large size malignant appearing gastric ulcer at the antrum, this was biopsied and computerized tomography (CT) of the thorax and abdomen was requested.
The CT Thorax and Abdomen scan showed thickening of the gastric antrum (Fig. 1) in additional to widespread lymphadenopathy in the greater omentum/mesentery (Fig. 2), the appearances were suggestive of gastric cancer. The CT scan also showed a coincidental finding of a left breast cancer (Fig. 3) with left axillary lymphadenopathy (Fig. 4). On further questioning the patient had been aware of the breast lump for 18 months but did not seek medical advice. Following the CT she underwent a mammogram which confirmed the left side breast cancer (Fig. 5).
Invasive ductal carcinoma grade 2 (Fig. 6). The Clinical tumour node metastasis (TNM) classification was T2, N1, M0.
The tumour cells are diffusely and strongly positive for oestrogen receptors, negative for progesterone receptors, positive for E-cadherin which is consistent with an invasive ductal carcinoma and Herceptin 2 receptors positive.
An adenocarcinoma of the stomach (Fig. 7) and a second opinion was sought with the Professor of Gastrointestinal histology who concluded an adenocarcinoma of the stomach. The tumour was oestrogen receptor negative. The clinical TNM classification was T2, N3, M0.
This was all discussed in the Multidisciplinary team meeting and it was that in evidence of oestrogen receptors being positive in the breast tumour and negative in the stomach tumour that the patient most likely had two primary tumours.
The breast tumour was treated with letrozole 2.5 mg daily as long term treatment. Surgical treatment was discussed with the patient however she wanted to await the outcome of her stomach cancer. Following that the patient then underwent a staging laparoscopy followed by a subtotal D2 gastrectomy with roux en Y reconstruction. At laparotomy it was found that the antral ulcer had invaded the pancreas over a 2 cm wide area, after further intraoperative consideration, the resection proceeded with excision of a cuff of pancreatic tissue. She made a good postoperative recovery.
Postoperatively the histology showed a large gastric ulcer with inflammation and fibrosis with NO evidence of malignancy. 22 lymph nodes were resected which were all negative for malignancy.
After the patient recovered from the gastrectomy, surgical treatment of her breast cancer was discussed again with the patient, however she refused and opted to continue with letrozole treatment. She has been seen regularly in the outpatient clinic following the operation and in her last clinic visit, which was approximately 14 months postoperatively, she has gained 16 kg in weight and the palpable tumour in her left breast has significantly reduced in size. She also expressed her satisfaction with her treatment up to date and is still not keen on surgical treatment, therefore the letrozole has been continued at 2.5 mg daily dose with a view of reviewing the patient in another 6 months. She had a CT scan of her abdomen which showed no evidence of recurrence of the gastric cancer.
The original gastric biopsies of the patient showing invasive adenocarcinoma of the stomach, which was confirmed by two independent histopathologists being ER receptor negative is more likely to be a primary gastric tumour. Unfortunately Immunohistochemical analysis could not be carried out as the samples were cut out of the block.
Irrespective of whether the gastric cancer was primary or metastatic from breast cancer, in our patient following the resection of the stomach, there was no evidence of malignancy in the stomach and none in the 22 resected lymph nodes. After careful analysis and review of the patient we have concluded that this could be due to three possibilities: 1) The only treatment instigated between diagnosis and resection of the gastric tumour was letrozole so, did letrozole have an effect on the gastric cancer. As we mentioned previously the main mechanism of action of letrozole is an aromatase inhibitor. We suggest that their might be other intracellular signaling pathways supported by evidence suggesting down regulation of genes associated with cell cycle and proliferation following letrozole treatment these changes could alter not only cellular expression but secondary consequences of cell death and clonal selection, in addition to longer term changes shown to alter vascularization, remodeling and the inflammatory response at the mitochondrial and extracellular matrix level.(14) This will need further research and studies to determine if letrozole could have a therapeutic effect on gastrointestinal tumours. 2) Did the gastric cancer undergo spontaneous remission? This is unlikely however there are a few published cases in literature and the mechanism is not clearly understood.(15) 3) Sampling error: Evidence of artefact or sampling error were investigated and double checked by two independent pathologists and discussed in the report and none were identified.
We would also like to highlight the importance of conducting Immunohistochemical analysis when patients are diagnosed with stomach cancer and are also found to have Breast cancer. Unfortunately in our case Immuno-histochemical analysis was not carried out as the samples were cut out of the block. As we have discussed earlier Immunohistochemical analysis is very useful in differentiating between primary stomach cancers from metastatic breast carcinoma of the stomach and hence the management of these patients.
In conclusion, a patient with oestrogen receptor positive breast cancer and oestrogen receptor negative gastric cancer was treated with letrozole and gastric resection. Independent histology by two pathologists pre-operatively diagnosed gastric adenocarcinoma. Post-operatively, independent analysis of the resected stomach, omentum, pancreas and lymph nodes revealed no evidence of gastric cancer. This suggests the possibility of letrozole having an effect on gastric cancer. Further studies are needed.
Figures and Tables
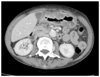 | Fig. 1CT abdomen showing thickening of the gastric antrum which measured approximately 19.3 mm. CT = computerized tomography. |
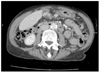 | Fig. 2CT abdomen demonstrates the mesenteric lymphadenopathy, showing two lymph nodes measuring 8.6 and 9.3 mm. CT = computerized tomography. |
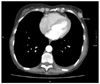 | Fig. 3CT thorax showing an incidental finding of a left side breast tumour measuring 18.2 mm as demonstrated on the scan. CT = computerized tomography. |
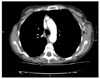 | Fig. 4CT thorax showing left axillary lymphadenopathy, one of the lymph node measured 11 mm as shown on the scan. CT = computerized tomography. |
 | Fig. 6Left breast biopsy (Formalin fixed paraffin embedded tissue stained with haematoxylin-eosin) showing invasive ductal carcinoma of the breast grade 2. |
Acknowledgments
We would like to thank the Pathology Department at the Aneurin Bevan Healthcare NHS trust, specially Dr. Elsie Wessels (Consultant Pathologist) for her help and valuable contribution with this case. We are also very grateful for Professor G Williams (Consultant Pathologist at the University Hospital of Wales) for his valuable opinion in regards to the diagnosis of this case.
References
1. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006. 354:270–282.

2. Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007. 105:Suppl 1. 7–17.

3. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003. 349:1793–1802.

4. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005. 97:1262–1271.

5. Tremblay F, Jamison B, Meterissian S. Breast cancer masquerading as a primary gastric carcinoma. J Gastrointest Surg. 2002. 6:614–616.

6. Yim H, Jin YM, Shim C, Park HB. Gastric metastasis of mammary signet ring cell carcinoma--a differential diagnosis with primary gastric signet ring cell carcinoma. J Korean Med Sci. 1997. 12:256–261.

7. Matsui M, Kojima O, Kawakami S, Uehara Y, Takahashi T. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992. 22:421–425.

8. van Velthuysen ML, Taal BG, van der Hoeven JJ, Peterse JL. Expression of oestrogen receptor and loss of E-cadherin are diagnostic for gastric metastasis of breast carcinoma. Histopathology. 2005. 46:153–157.

9. Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT. Gross cystic disease fl uid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. 1989. 20:281–287.

10. Mazoujian G, Pinkus GS, Davis S, Haagensen DE Jr. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol. 1983. 110:105–112.
11. Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007. 127:103–113.
12. O'Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med. 2005. 129:338–347.
13. Jones GE, Strauss DC, Forshaw MJ, Deere H, Mahedeva U, Mason RC. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol. 2007. 5:75.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download