Abstract
Mesenteric fibromatosis is a monoclonal, fibroblastic proliferation arising from musculoaponeurotic structure, and it is distinctive lesions defined as a group of non-metastasizing fibroblastic tumors which has local invasion and has a high recurrence rate after the surgical excision. The main treatment modality is the surgical excision. Radiation therapy, chemotherapy, and hormone therapy are also known as useful treatments. We report our experience of a recent case of Mesenteric fibromatosis. A 62-year old female patient had undergone gastrectomy due to gastric cancer. 18 months after gastrectomy, we detected an abdominal mass. The preoperative radiologic findings were suggestive of recurrence. Exploratory laparotomy was performed and post-operative pathologic diagnosis was confirmed as fibromatosis. We report a patient with mesenteric fibromatosis that mimic recurrence after gastrectomy for gastric cancer.
Figures and Tables
Fig. 1
Abdominal CT (computed tomography) finding. Abdominal CT shows a 4.5 cm sized new round soft-tissue mass in mesentery of left lower abdomen.
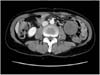
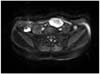
Fig. 2
MRI (magnetic resonance image) finding. MRI shows about 5.3×3.2 cm sized ovoid delayed enhancing heterogeneous signal intensity mass in the left lower abdomen on T2 weighed image.
References
1. Allen PW. The fibromatoses: a clinicopathologic classification based on 140 cases. Am J Surg Pathol. 1977. 1:255–270.
2. Dong-Heup K, Kim DH, Goldsmith HS, Quan SH, Huvos AG. Intra-abdominal desmoid tumor. Cancer. 1971. 27:1041–1045.

3. Reitamo JJ, Häyry P, Nykyri E, Saxén E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the finnish population. Am J Clin Pathol. 1982. 77:665–673.

4. Burke AP, Sobin LH, Shekitka KM, Federspiel BH, Helwig EB. Intra-abdominal fibromatosis. A pathologic analysis of 130 tumors with comparison of clinical subgroups. Am J Surg Pathol. 1990. 14:335–341.
5. Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007. 25:1785–1791.

6. Reitamo JJ, Scheinin TM, Häyry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986. 151:230–237.
8. Lynch HT, Fitzgibbons R Jr. Surgery, desmoid tumors, and familial adenomatous polyposis: case report and literature review. Am J Gastroenterol. 1996. 91:2598–2601.
9. Simpson RD, Harrison EG Jr, Mayo CW. Mesenteric fibromatosis in familial polyposis. A variant of Gardner's syndrome. Cancer. 1964. 17:526–534.

10. Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004. 100:612–620.

11. Brooks AP, Reznek RH, Nugent K, Farmer KC, Thomson JP, Phillips RK. CT appearances of desmoid tumours in familial adenomatous polyposis. Further observations. Clin Radiol. 1994. 49:601–607.

12. Pickhardt PJ, Bhalla S. Unusual nonneoplastic peritoneal and subperitoneal conditions: CT findings. Radiographics. 2005. 25:719–730.

13. Lee JC, Thomas JM, Phillips S, Fisher C, Moskovic E. Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol. 2006. 186:247–254.

14. Lo KW. Mesenteric fibromatosis as a potential source of false-positive interpretation of FDG-PET: report of a case. Dis Colon Rectum. 2007. 50:924–926.

15. Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol. 2007. 80:750–756.

16. Posner MC, Shiu MH, Newsome JL, Hajdu SI, Gaynor JJ, Brennan MF. The desmoid tumor. Not a benign disease. Arch Surg. 1989. 124:191–196.
17. Spear MA, Jennings LC, Mankin HJ, Spiro IJ, Springfield DS, Gebhardt MC, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys. 1998. 40:637–645.

18. Lahat G, Nachmany I, Itzkowitz E, Abu-Abeid S, Barazovsky E, Merimsky O, et al. Surgery for sporadic abdominal desmoid tumor: is low/no recurrence an achievable goal. Isr Med Assoc J. 2009. 11:398–402.
19. Reitamo JJ. The desmoid tumor. IV. Choice of treatment, results, and complications. Arch Surg. 1983. 118:1318–1322.
20. Melis M, Zager JS, Sondak VK. Multimodality management of desmoid tumors: how important is a negative surgical margin? J Surg Oncol. 2008. 98:594–602.

21. de Camargo VP, Keohan ML, D'Adamo DR, Antonescu CR, Brennan MF, Singer S, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer. 2010. 116:2258–2265.

22. Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006. 24:102–105.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download