Abstract
Purpose
This study was done to evaluate the usefulness of preoperative computed tomography (CT) and intraoperative laparoscopic ultrasound to facilitate treatment of gastric submucosal tumors.
Materials and Methods
The feasibility of laparoscopic wedge resection as determined by CT findings of tumor size, location, and growth pattern was correlated with surgical findings in 89 consecutive operations. The role of laparoscopic ultrasound for tumor localization was analyzed.
Results
Twenty-three patients were considered unsuitable for laparoscopic wedge resection because of large tumor size (N=13) or involvement of the gastroesophageal junction (N=9) or pyloric channel (N=1). Laparoscopic wedge resection was not attempted in 11 of these patients because of large tumor size. Laparoscopic wedge resection was successfully performed in 65 of 66 (98.5%) patients considered suitable for this procedure. Incorrect interpretation of preoperative CT resulted in a change of surgery type in seven patients (7.9%): incorrect CT diagnosis on gastroesophageal junction involvement (N=6) and on growth pattern (N=1). In 18 patients without an exophytic growth pattern, laparoscopic ultrasound was necessary and successfully localized all lesions.
Gastric submucosal tumors (SMTs) encompass both non-neoplastic and neoplastic conditions of various etiologies, which arise in the deep layers of the gastric wall (submucosa or muscularis propria). Additionally, some strictly mucosal lesions, such as carcinoid tumors or leiomyomas originating from the muscularis mucosa, are included in the "submucosal" category by convention. (1) Gastrointestinal stromal tumors (GISTs) are by far the most frequently encountered submucosal malignancy. Current preoperative diagnostic modalities have limitation for differentiating GISTs from other benign SMTs.(2) Therefore, surgical excision of the lesions is recommended in cases of gastric SMTs with suspicious malignant potentials or symptomatic lesions.
Recently, laparoscopic wedge resection has been regarded as the treatment of choice for gastric submucosal tumors with relatively small size because of its less invasiveness compared to the traditional open approach.(3,4) However, more extensive surgery (proximal, distal, and total gastrectomy) should be performed in cases with gastroesophageal junction or pyloric channel involvement, or with large tumors.(5)
Several laparoscopic wedge resection techniques have been described in the literature.(6-10) Although the surgical approach should be tailored, no comprehensive indications for the application of these techniques have been suggested. For the tailored application of surgical approaches, it is critical to preoperatively estimate the characteristics of each tumor, including size, involvement of gastroesophageal junction or pylorus, and presence of an exophytic component. Furthermore, in cases without exophytic components, accurate localizing procedures should be performed intraoperatively for laparoscopic resections.(11) Although intraoperative endoscopy is widely performed technique to locate small lesions,(12) it is a complex procedure, requiring experience and specific instruments in the operating room and is also associated with unwanted distension of upper gastrointestinal tract, resulting in the compromise of visual filed and laparoscopic manipulation of stomach.(13)
Over the past decade, multidetector computed tomography (CT) has been developed for the preoperative evaluation of gastric neoplasms,(14,15) and the utility of intraoperative laparoscopic ultrasound (LUS) for tumor localization during gastric resection has been also mentioned in several case reports.(8,11,16) Thus, the purpose of our study was to evaluate the utility of image-based surgical approaches using preoperative CT and intraoperative LUS to facilitate minimally invasive treatment of gastric submucosal tumors.
From January 2003 to December 2007, eighty-nine consecutive patients with gastric submucosal tumors detected on endoscopy and CT were referred for surgical treatment. We retrospectively analyzed 89 consecutive gastric SMT resections using a prospectively collected database. Informed consent was obtained from all patients prior to endoscopy, CT scanning, and surgery. surgery for gastric SMT were as following; 1) a tumor size larger than 2 cm, 2) a tumor with symptom of obstruction or ulcer with bleeding regardless of size of the tumor, or 3) a growing tumor confirmed by serial follow-up endoscopy or CT scanning, even if the tumor size was less than 2 cm.
Endoscopy of the upper gastrointestinal tract was performed by attending gastroenterologists during the preoperative evaluation. End-viewing fiberoptic panendoscopes (GIF-Q260, GIF-H260; Olympus, Tokyo, Japan) were used after intravenous administration of 5 mg midazolam (Taro Pharmaceutical International, Yakum, Israel) with the use of standard practices.
CT scans were subsequently performed using one of three multisection CT scanners (4-, 16- or 64-channel multi-detector row CT) (Lightspeed Plus, GE Medical Systems, Milwaukee, WI, USA; Sensation 16 and 64, Siemens Medical Solutions, Forchheim, Germany) for preoperative diagnosis. The patients fasted for at least six hours prior to the examination. Our CT protocol was changed during the study period. Initially, nine patients underwent routine abdominal CT in the supine position (450 ml oral contrast agent, N=5, no oral contrast, N=4). The next 80 patients underwent stomach-dedicated CT protocols and drank 600~800 ml of water as an oral contrast agent (N=26) or ingested 8 g of effervescent granules (Top; Taejoon Pharmaceutical, Seoul, Korea) with 10 ml of tap water (N=53) to distend the stomach immediately before scanning. Scanning positions were variable and unrelated to the tumor location (prone: N=18, and supine: N=61). Contrast medium with an iodine concentration of 370 mg I/ml (Ultravist 370, Schering) was administered using a power injector at a rate of 3~4 ml/sec through an 18-gauge plastic IV catheter placed in an antecubital vein. For both gastric and routine CT protocols, biphasic CT scans were obtained using an automatic bolus-tracking technique. Arterial phase scanning began automatically from the diaphragmatic dome to the level of the iliac crest, ten seconds after the trigger threshold (100 HU) was reached. Images of the portal phase were obtained twenty seconds after arterial phase scanning from the diaphragmatic dome to the level of the symphysis pubis. Image reconstruction of axial and coronal images was performed with 3- or 5-mm section thickness at 3- or 5-mm intervals.
All images of preoperative exams were interpreted on a picture archiving and communication system (PACS) workstation (Centricity; General Electric Medical Systems, Milwaukee, WI, USA), which allows interactive analysis. One radiologist and one surgeon prospectively evaluated the CT images together with reference to endoscopic findings and obtained consensus results. The location, growth pattern, and size of each lesion were recorded. The location was divided grossly into the following regions: cardia, fundus, body and antrum. The growth pattern was divided into two groups according to the presence or absence of an exophytic component. The size was measured as the longest diameter of the mass in the axial or coronal image using the electronic caliper on PACS workstation.
On the basis of CT findings with reference to endoscopic images, we selected the candidate group for laparoscopic wedge resection. The classification criteria were as follows. First, we considered that large lesions were not suitable for laparoscopic wedge resection because they sometimes require more extensive surgery.(5) The size limitation of 7 cm was made because the necessity of a long incision for the removal of the tumor decreases the advantage of the laparoscopic approach over open surgery, and the possibility of tumor breakage during laparoscopy is increased for larger tumors. Second, lesions located less than 1 cm from the gastroesophageal junction or the pyloric ring were considered unsuitable for laparoscopic wedge resection.(17) Hence, we visually estimated the distance between gastric extremities and the margin of the tumor (cardia or upper body location, from the gastroesophageal junction and proximal margin of the tumor; antral location, from the pyloric ring and distal margin of the tumor) on preoperative CT imaging. In equivocal cases, we estimated the distance using an electronic cursor on the PACS system or using oblique planar reformatted images displaying the shortest distance between the gastric extremities and the tumor margin (Fig. 2).
The candidates for laparoscopic wedge resection were classified into subgroups with or without the exophytic component based on CT findings. In cases with an exophytic component, an extraluminal wedge resection was planned. However, in cases without the exophytic component, we applied a transgastric approach for lesions located in the posterior wall and an eversion technique for lesions located in the anterior wall under the guidance of LUS localization, which was used because the laparoscopy alone could not localize the lesion.
Based on the CT findings, an open surgical approach was performed using the standard method for cases with large tumor mass; the laparoscopic approach was not attempted. All lesions less than 7 cm were approached laparoscopically because they can be removed through 5 cm incisions usually used for laparoscopy. Even in cases with lesions closer than 1 cm from the gastric extremities as determined by preoperative CT imaging, a surgeon with expertise in laparoscopic surgery assessed the feasibility of laparoscopic wedge resection, and sometimes attempted the resection because a negative microscopic margin guarantees curative resection of gastrointestinal stromal tumors.(18)
For laparoscopic surgery, three trocars were usually used; first, one 10 mm trocar was placed at the umbilicus using the open technique for a camera port. One 12 mm trocar was placed at the right midclavicular line about 2 cm above the level of umbilicus and the other 12 mm trocar was placed at the right subcostal area; both were placed under laparoscopic view after establishing the pneumoperitoneum. An additional 5 mm trocar was placed at the left subcostal area, if needed. After that, the laparoscopic survey to detect the exophytic component of tumors was performed for localization. If the exophytic components were detected on laparoscopy, extraluminal wedge resection was performed using linear endo-staplers. Otherwise, LUS was performed by one radiologist (Lim JS) with 7 years of experience for abdominal imaging-based tumor localization. Immediately before the examinations, percutaneous aspiration with a 9 cm 19 gauge spinal needle was performed to eliminate air from the stomach, as nasogastric tubes were not inserted in all patients.(19) Removal of air from the stomach also improves the sonic window for LUS. After needle decompression, the LUS probe (Aloka Co. Ltd., Tokyo, Japan) was inserted into the abdominal cavity via the 12 mm port at the right mid-clavicular line. The laparoscopic ultrasound examination was performed with small, slow, transversal, and rotating movements at the anterior gastric wall to explore the presumed site for gastrotomy based on the transgastric approach or eversion method.(6,8,16)
All laparoscopic procedures were performed without any open conversion. There were 51 females and 38 males with a mean age of 54 years (range 22~80). The resected tumors were located as follows: cardia, N=10; cardia and body, N=8; fundus, N=17; body, N=44; antrum, N=10. The mean tumor size was 4.5 cm (range, 0.8~28 cm). Histopathological examination showed 53 gastrointestinal stromal tumors, 11 schwannomas, 10 ectopic pancreases, 9 leiomyomas, and 6 miscellaneous tumors (Table 1). The pathologic reports showed negative microscopic margins for all tumors. The mean length of postoperative hospital stay was 4.7 days (range 1~70 days). The mean hospital stay of patients who received laparoscopic wedge resection (N=74) was 4.1 days (range 1~70 days) while that of patients who underwent any types of laparoscopic gastrectomy (N=15) was 7.5 days (range 5~13 days). There were three postoperative complications (3.4%) including two wound complications and a leakage from the staple line after laparoscopic wedge resection.
Results from the image-based approach are shown in Fig. 4 and 5. According to our criteria for laparoscopic wedge resection, 23 patients were considered unsuitable based on preoperative CT imaging results (Fig. 4). Thirteen patients were excluded due to tumor sizes over 7 cm (range 7.1~28 cm). Open surgery was planned and performed in 11 patients with large tumors. However, two patients with tumor sizes of 7.3 and 7.4 cm were treated by laparoscopic wedge resection because these patients wanted laparoscopic operation. Nine patients were considered not to be suitable for laparoscopic wedge resection due to gastroesophageal junction involvement closer than 1 cm. Three patients underwent laparoscopic gastrectomy as predicted (proximal subtotal gastrectomy, N=2; total gastrectomy, N=1). However, laparoscopic wedge resections were possible for the remaining six patients. One patient could not undergo laparoscopic wedge resection due to pyloric channel involvement and instead underwent laparoscopic distal subtotal gastrectomy.
Sixty-six patients were considered to be suitable for laparoscopic wedge resection based on CT imaging (Fig. 5). Of these patients, the diagnosis of the presence or absence of the exophytic component was correct in 65 (65/66). Exophytic components were detected on CT imaging in 28 patients (28/66). These patients all underwent laparoscopic wedge resections without additional localizing procedures. The other 38 patients (38/66) did not have exophytic components based on CT findings. For 18 patients without exophytic components (18/38), the accurate site of gastrotomy for the transgastric or eversion techniques could not be determined from the laparoscopic findings alone. LUS was thus performed to localize the lesions, especially for patients with small lesions. Tumor and gastrotomy sites were successfully localized by LUS in all 18 cases. None of these patients needed intraoperative endoscopy for localization. The mean size of these lesions on CT images was 2.6 cm (range 1.8~4.8 cm). The mean procedure duration for LUS was 107 seconds (range 15~330 seconds). Laparoscopic wedge resections were successfully performed in 65 patients (extraluminal, N=41; transgastric, N=19; eversion, N=6). The remaining patient underwent laparoscopic total gastrectomy due to the risk of luminal compromise at the gastroesophageal junction despite the suitability of laparoscopic wedge resection based on preoperative CT imaging. In addition, of the 65 patients who underwent laparoscopic wedge resection, incorrect CT interpretation of the lesion as having no exophytic component resulted in an unnecessary anterior wall gastrotomy in one patient.
Our image-based surgical approach was clinically feasible and useful. We considered the characteristics of the tumor, i.e., size, location, and growth pattern, as criteria for applying different types of surgery. The feasibility of laparoscopic wedge resection might be successfully predicted by preoperative interpretation of tumor characteristics based on CT findings. The need for further localization procedures such as LUS was successfully predicted by analyzing the growth pattern of the lesions on CT findings. In lesions without an exophytic component, LUS was effective for localizing gastric submucosal tumors for laparoscopic surgery. With this technique, laparoscopic tumor resection could be successfully performed by prompt intraoperative localization in the surgical field.
Laparoscopic wedge resection for gastric submucosal tumors is regarded as the treatment of choice, but cannot be applied uniformly. The surgeon should consider characteristics of the gastric submucosal tumor such as the size, location, and growth pattern. There are several techniques for laparoscopic wedge resection. The first is the extraluminal wedge resection technique for exophytic lesions; this technique is optimal because accurate localization is possible only with laparoscopy.(10) The second is the transgastric approach, which is commonly used for posterior wall intraluminal lesions and requires an anterior gastrotomy.(6) The third is the eversion method used for tumors with intraluminal growth patterns.(8) Difficulties in tumor resection are mainly caused in intraluminal lesions because on laparoscopy, the tumors cannot be detected from the serosal side.(17) Therefore, tumors without exophytic components require additional procedures for intraoperative localization.
The current standard of preoperative work-up for patients with gastric submucosal tumors includes endoscopy, endoscopic ultrasound, barium study, and CT. Over the past decade, CT has been used to diagnose the presence of gastric submucosal tumors and possible metastatic foci, and for the evaluation of gastric neoplasms. (14,16) Particularly during preoperative evaluation of gastric submucosal tumors, CT can provide accurate information about tumor size, presence or absence of exophytic component, and the distance from the tumor margin to the gastroesophageal junction or pylorus, whereas endoscopy and barium analyses can only provide rough information. These are important factors for surgical planning; therefore, we tried to determine the surgical approach by evaluating the CT findings using previously established criteria for laparoscopic wedge resection.
Laparoscopic wedge resection should be avoided for large tumors or those near the gastric extremities due to the difficulty of removal and the risk of clinically significant deformity or stenosis.(17) CT can provide information about the size of the tumor and enables the evaluation of the gastric extremities.(20) In our study, we successfully applied the laparoscopic approach to patients with tumors of less than 7 cm on CT imaging without conversion to open surgery. Additionally, we performed laparoscopic wedge resection for two patients with tumors larger than 7 cm (7.3 and 7.4 cm) because they strongly laparoscopic surgery. Although the application of laparoscopic methods to large gastrointestinal stromal tumors is controversial, in this study we successfully performed laparoscopic wedge resection without rupture of the tumor in all cases.
In cases with gastroesophageal junction or pyloric involvement revealed by preoperative CT, laparoscopy-assisted gastrectomy was performed after intraoperative determination of the possibility of deformity or stenosis after resection of the tumor. In this process, preoperative CT imaging of gastroesophageal junction involvement was somewhat problematic (incorrect prediction: total number=7; not suitable, N=6; suitable, N=1). There are several possible explanations for the incorrect evaluations. First, insufficient distension might result in incorrect estimations of the distance between the tumor and gastroesophageal junction seems to be closer than what was actually found. In our study, 600~800 ml water or 8 g effervescent granules were usually ingested for distention of the gastric lumen. Larger amount of water or gas distention could be helpful for overcoming this problem. Additionally, the scanning position was determined without respect to the tumor location. Determining the optimal scanning position according to the tumor location would be helpful for gastric distention (antral location, prone; fundal location, supine). Second, the surgical technique changed. During the initial period of our study, laparoscopic staplers were routinely used for simultaneous tumor excision and gastric wall suturing. However, later in the study, excision and suturing techniques were introduced in cases where the tumor was located near gastroesophageal junction. This procedure might enable laparoscopic wedge resection in cases with a preoperative estimated distance of less than 1 cm.
The preoperative identification of exophytic components on CT imaging is important for surgical planning. For tumors with exophytic components, extraluminal wedge resection is optimal without gastrotomy because accurate localization is possible with laparoscopy alone.(10) In our study, CT imaging successfully revealed exophytic components in all 28 cases intraoperatively confirmed to have the exophytic components. These patients all underwent successful laparoscopic extraluminal wedge resections. On the other hand, tumors without exophytic components could present an obstacle to laparoscopic wedge resection. In particular, small lesions are more difficult to localize due to the absence of tactile sensation during laparoscopic surgery. The location must be precisely identified immediately before resection is undertaken. However, preoperative imaging (endoscopy, upper barium study and CT) can only provide rough information about the lesion, and its location can vary based on the degree of gastric distension during the study.(13,20) Methods such as endoscopic tattooing, intraoperative enteroscopy and LUS are used to solve this problem.(13,21,22) LUS was already suggested in our previously published report as an alternative to preoperative endoscopic tattooing and intraoperative endoscopic localization, which can be time-consuming and result in procedure-related complications.(11) In this study, LUS localization of tumors was prompt (mean procedure duration: 107 sec) despite the small size of most of the tumors (mean size: 2.6 cm). Even in the case of large tumor, LUS may sometimes be helpful for laparoscopic wedge resection. Although the experienced surgeons may roughly localize the tumor with the laparoscopic palpation, determination of exact gastrotomy site may be difficult. Accurate selection of gastrotomy site guided by LUS can reduce unnecessary resection of the gastric wall for the margin and also minimize of the length of gastrotomy.(8)
There are limitations to our study. First, our CT protocol was not standardized because the CT images had been already obtained when patients were enrolled after surgical consultation. Thus, 9 of our 89 patients did not undergo CT with water or air as an oral contrast agent. Insufficient gastric distention in those cases might have resulted in an underestimation of the distance between the gastric extremities and the proximal margin of the tumor. However, none of these nine cases showed a distance of less than 1 cm from the gastric extremities. Second, CT may have generally underestimated the distance due to intrinsic gastric peristalsis, elasticity, and insufficient gastric distention. The 1 cm criterion itself is also not absolute; it may vary according to tumor location (anterior or posterior wall origin) or the applied surgical technique. Third, not all hospitals or surgeons have LUS or experienced radiologists; thus the feasibility of the image-based approach might be influenced by the experience of the surgeon. Lastly, we could not include preoperative endoscopy or endoscopic ultrasound, which are very important diagnostic tools, because the main purpose of the modalities at the time of performance was diagnosis or surgical decision. The detailed selection of surgical technique was not main concern. Furthermore, the identification of exophytic component is not possible in case of endoscopy and the retrospective analysis for distance measurement from gastric extremities was not possible on the basis of only captured images of the modalities.
In conclusion, image-based laparoscopic wedge resection was clinically feasible and useful for treatment of gastric submucosal tumors, although the preoperative evaluation of gastric extremities needs improvement. Preoperative CT imaging might be helpful for determining the type of surgery required. In addition, for tumors lacking an exophytic component, laparoscopic ultrasound is effective for localizing lesions for laparoscopic wedge resection, especially for small endoluminal submucosal tumors.
Figures and Tables
Fig. 1
Image-based approach for laparoscopic wedge resection. SMT = submucosal tumor; LUS = laparoscopic ultrasound.
Fig. 2
Estimation of the involvement of the gastric extremities. (A) A 49-year-old woman with pathologically confirmed true leiomyoma. Gastroesophageal junction involvement was found (arrow). Thus, this patient underwent laparoscopic total gastrectomy. (B) A 69-year-old man with pathologically confirmed gastrointestinal stromal tumor. This patient underwent laparoscopic subtotal gastrectomy due to pyloric channel involvement of the tumor (white and black arrows). (C) A 46-year-old woman with pathologically confirmed gastrointestinal stromal tumor (long arrow) in the gastric upper body. The distance between the gastroesophageal junction and the proximal tumor margin was measured as 2.1 cm (short arrow). This patient was regarded as acceptable for laparoscopic wedge resection and underwent the surgery.

Fig. 3
Use of laparoscopic ultrasound for laparoscopic localization. (A) CT imaging demonstrated a 1.5-cm intraluminal lesion without exophytic component in gastric fundus (arrow). (B) The lesion was not detected by laparoscope. LUS probe were searching for the lesion. (C) It was easily detected by laparoscopic ultrasound (arrow). (D) Gastrotomy was performed with a dissector at the site localized by laparoscopic ultrasound. The gastrotomy site accurately coincided with the lesion (arrow). The lesion was easily resected. CT = computer tomography; LUS = laparoscopic ultrasound.
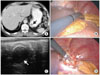
Fig. 4
Intraoperative results of 23 patients deemed not suitable for laparoscopic wedge resection based on preoperative CT findings. CT = computed tomography.

References
1. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005. 37:635–645.

2. Kang JH, Lim JS, Kim JH, Hyung WJ, Chung YE, Choi JY, et al. Role of EUS and MDCT in the diagnosis of gastric submucosal tumors according to the revised pathologic concept of gastrointestinal stromal tumors. Eur Radiol. 2009. 19:924–934.

3. Choi YB, Oh ST. Laparoscopy in the management of gastric submucosal tumors. Surg Endosc. 2000. 14:741–745.

4. Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002. 16:803–807.

5. Avital S, Brasesco O, Szomstein S, Liberman M, Rosenthal R. Technical considerations in laparoscopic resection of gastric neoplasms. Surg Endosc. 2003. 17:763–765.

6. Hepworth CC, Menzies D, Motson RW. Minimally invasive surgery for posterior gastric stromal tumors. Surg Endosc. 2000. 14:349–353.

7. Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008. 22:1729–1735.

8. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Noh SH. Laparoscopic resection of a huge intraluminal gastric submucosal tumor located in the anterior wall: eversion method. J Surg Oncol. 2005. 89:95–98.

9. Ibrahim IM, Silvestri F, Zingler B. Laparoscopic resection of posterior gastric leiomyoma. Surg Endosc. 1997. 11:277–279.

10. Otani Y, Ohgami M, Igarashi N, Kimata M, Kubota T, Kumai K, et al. Laparoscopic wedge resection of gastric submucosal tumors. Surg Laparosc Endosc Percutan Tech. 2000. 10:19–23.

11. Hyung WJ, Lim JS, Cheong JH, Lee YC, Noh SH. Tumor localization using laparoscopic ultrasound for a small submucosal tumor. J Surg Oncol. 2004. 86:164–166.

12. Ohgami M, Otani Y, Kumai K, Kubota T, Kim YI, Kitajima M. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg. 1999. 23:187–192.

13. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc. 2005. 19:1353–1357.

14. Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003. 23:625–644.

15. Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003. 23:75–87.

16. Santambrogio R, Montorsi M, Schubert L, Pisani Ceretti A, Costa M, Moroni E, et al. Laparoscopic ultrasound-guided resection of gastric submucosal tumors. Surg Endosc. 2006. 20:1305–1307.

17. Aogi K, Hirai T, Mukaida H, Toge T, Haruma K, Kajiyama G. Laparoscopic resection of submucosal gastric tumors. Surg Today. 1999. 29:102–106.

18. Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, et al. NCCN Task Force. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007. 5:Suppl 2. S1–S29.

19. Hyung WJ, Song C, Cheong JH, Choi SH, Noh SH. Percutaneous needle decompression during laparoscopic gastric surgery: a simple alternative to nasogastric decompression. Yonsei Med J. 2005. 46:648–651.

20. Lee MW, Kim SH, Kim YJ, Lee JM, Lee JY, Park EA, et al. Gastrointestinal stromal tumor of the stomach: preliminary results of preoperative evaluation with CT gastrography. Abdom Imaging. 2008. 33:255–261.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download