Introduction
Gastrointestinal stromal tumors (GISTs) constitute the most common primary mesenchymal tumors of the digestive tract and characteristically express c-kit (CD117) in most cases.(1-3) Most common non-epithelial tumor of the GI tract, often originate from the stomach & small bowel but accounting for only 1% of GI malignancy. Particularly, synchronous occurrence of GIST with other epithelial tumor is rarely reported. Concurrent small bowel GIST and gastric cancer is very unusual.
We report a case of a synchronous occurrence of gastric cancer and small bowel GIST that was suspected with the peritoneal seeding of the gastric cancer.
Case Report
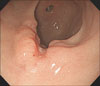
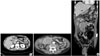
A 55 year-old man with diabetes was admitted to the Gastroenterology department of Kyung Hee University Hospital on December 28, 2008, with a history of intermittent cramping epigastric pain. On examination, the patient's abdomen was soft and was not distended, but a painless abdominal mass was palpated in the right upper guardant. No tenderness was eliciteded in the other abdominal areas. Complete blood count showed hemoglobin 12.5 g/dl; white blood cell count, 14,540/µl (seg., 80.6%; lymphocyte, 13.3%; eosinophils, 1.4%); and platelet count, 774,000/µl. Tumor markers were unremarkable (CEA 0.59 ng/ml, CA19-9 4.19 U/ml, AFP 3.97 ng/ml). Gastroscopy revealed the advanced gastric cancer in the anterior wall of the antrum and biopsy confirmed poorly differentiated adenocarcinoma (Fig. 1). Computed tomography (CT) of the abdomen revealed wall thickening in the gastric antrum, as well as omental and mesenteric metastatic masses. Suspected peritoneal seeding secondary to cancer infiltration was shown in the right omentum and wall thickening at hepatic flexure of the colon (Fig. 2). The patient underwent laparoscopic examination followed by distal gastrectomy with D2 lymph node dissection and segmental resection of the small intestine. Intra-operatively, the abdominal mass was suspected to be small bowel GIST was and not gastric cancer metastasis. The gastric lesion was in the antrum associated with ulceration, measuring 2×1.5 cm. It invaded the submucosa without metastasis in all 11 regional lymph nodes (Fig. 3A). H&E staining of the gastric lesion showed poorly differentiated adenocarcinoma with signet ring cells (Fig. 4A). The small bowel lesion on the serosal surface was a multi-lobulated mass, measuring 8×7×7 cm. The cut surface of mass showed a brownish solid area with focal hemorrhagic and necrotic areas (Fig. 3B). Histologic examination of the resected small intestine portion showed a well-circumscribed tumor consisted of interlacing or whorling of spindle cells with intervening hyalinized stroma (Fig. 4B), associated with increased cellularity and frequent mitosis (more than 8/50 HPFs). Necrosis was also seen. Regional lymph node showed reactive changes. Immunohistochemical staining of the small bowel mass is positive for CD117 (Fig. 4C). After considering all the pathologic results, the small-intestinal tumor was diagnosed as high-risk gastrointestinal stromal tumor (GIST).
The Patient was discharged in 10 days after the operation without complications. He refused the adjuvant therapy for GIST. As of 29 December, the patient showed no other marked indispositions and has remained disease-free for 14 months.
Discussion
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract. They are defined here as KIT (CD117, stem cell factor receptor)-positive mesenchymal spindle cell or epithelioid neoplasms in the GI tract, omentum, and mesentery. GISTs typically present in older individuals and are most common in the stomach (60~70%), followed by small intestine (20~25%), colon and rectum (5%), and esophagus (<5%). Benign tumors outnumber the malignant ones by a wide margin. Approximately 70% of GISTs are positive for D34, 20~30% are positive for smooth muscle actin (SMA), 10% are positive for S100 protein and <5% are positive for desmin.
The gastrointestinals stromal tumors represent a very rare group of digestive tract tumors, with malignant evolution potential. The treatment choice is surgery, with complete ablation of the tumor. Medical treatment with imatinib (Glivec®, Novartis AG, Basel, Switzerland) is necessary for recurrence or metastases. Stromal tumors undergo unusual evolution, and for this reason all patients must be under medical observation, for the rest of their life. Simultaneous occurrence of GIST and early gastric cancer is very uncommon. To the best of our knowledge only one case has been reported.(3) Ozgun et al. reported the first case of GIST synchronous with primary gastric(4) adenocarcinoma in a patient presented with bowel perforation in 2009. We found synchronous gastric cancer and small bowel GIST that was initially suspected to be gastric cancer peritoneal seeding. We found various hypotheses on the synchronous occurrence of stromal tumors and adenocarcinomas.(5) It is possible that the same theories are valid for simultaneous occurrence of GIST and early gastric cancer. such as being male, Helicobacter pylori infections, germline mutations, and exposure to ionizing radiation.
It is not known whether this association is a simple incidental co-existence or whether it is casually connected. More investigations are needed to elucidate this further.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download